Promising Outcomes from Transcenta's Osemitamab Triple Trial in G/GEJ Cancer at ESMO 2024
Transcenta Holding Limited ("Transcenta") (HKEX: 06628), an internationally active clinical-stage biopharmaceutical company specializing in the discovery, research, development, and production of antibody-based therapeutics, has released updated information regarding the cohort-G results for Osemitamab (TST001) in combination with Nivolumab and CAPOX as a first-line therapy for patients with advanced G/GEJ cancer (TranStar102). The latest data reaffirms the promising efficacy observed in the previously reported findings at ASCO 2024.
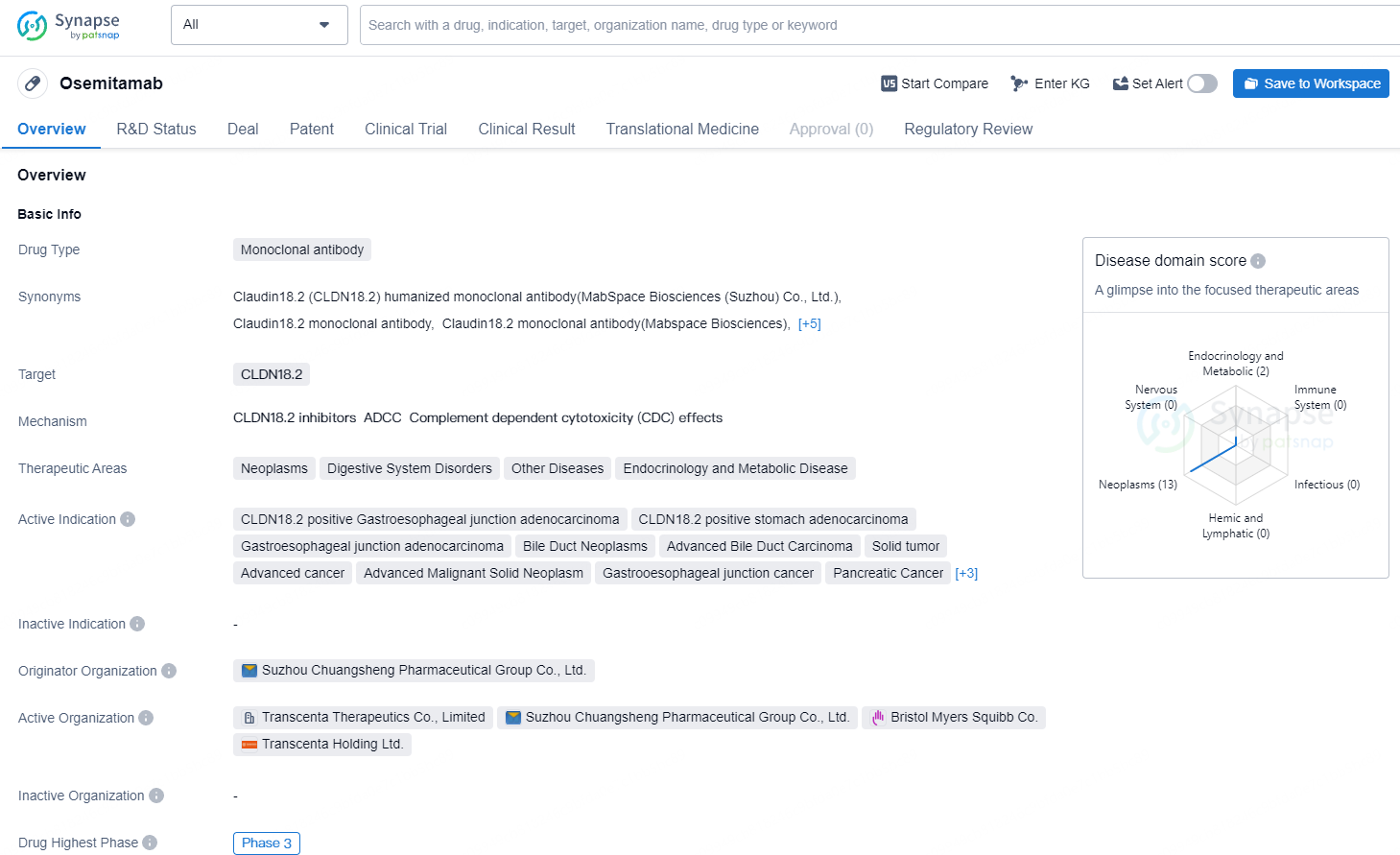
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The findings indicated that among patients with identified CLDN18.2 and PD-L1 statuses, those exhibiting high or medium (H/M) CLDN18.2 expression achieved a median progression-free survival (mPFS) of 14.2 months, alongside a confirmed objective response rate of 68%. Most of these patients had a PD-L1 combined positive score (CPS) of less than 5.
In a comparative analysis using a control group with very low or no CLDN18.2 expression, the hazard ratio (HR) for the triple therapy was 0.505 (95% CI, 0.244-1.045), favoring patients with H/M CLDN18.2 expression, irrespective of CPS level. This data supports the synergistic interaction between the CLDN18.2-targeting agent and checkpoint inhibitors, as previously reported by Transcenta at ESMO last year, which demonstrated that the CLDN18.2-targeting antibody can upregulate PD-L1 expression in gastric cancer cells and promote T-cell infiltration. As of the analysis cutoff, the median overall survival (OS) had not been achieved due to a limited number of events; however, the 12-month survival rate for the 82 patients in this cohort was 73.8% (95% CI: 62.0-82.4%).
Previous research has established that the Zolbetuximab plus CAPOX combination in CLDN18.2-positive patients enhances PFS from 6.80 to 8.21 months (HR = 0.687; 95% CI, 0.544-0.866) (Source: Shah, Manish A et al. Nature Medicine 2023 Aug 29 (8): 2133-2141). The updated data from Cohort-G indicates that the triple combination of Osemitamab (TST001), Nivolumab, and CAPOX performs significantly better compared to historical data of Nivolumab plus CAPOX or Zolbetuximab plus CAPOX combinations.
This updated data was presented as a poster (Abstract #1419p) on September 16, 2024, at the ESMO Congress 2024 in Barcelona, Spain.
“The recent results from the Cohort-G trial of Osemitamab (TST001) with Nivolumab and CAPOX for first-line treatment in advanced gastric/gastroesophageal junction (G/GEJ) cancer are very promising. The high objective response rate and extended median progression-free survival in patients with high/medium CLDN18.2 expression and known PD-L1 status compared to those with no CLDN18.2 expression underscore the effectiveness of combining Osemitamab (TST001) with checkpoint inhibitors, even in low PD-L1 expressers,” stated Dr. Caroline Germa, Executive Vice President, Global Medicine Development and Chief Medical Officer at Transcenta. “These findings showcase the potential of Osemitamab (TST001) in enhancing treatment outcomes for advanced G/GEJ cancer patients.”
“Data from Cohort-G presented at ESMO 2024 clearly demonstrate the clinical benefits of the triple therapy combination. The notable improvements in progression-free survival and objective response rate in patients with high/medium CLDN18.2 expression and low PD-L1 CPS emphasize the promise of this therapeutic strategy,” stated Professor Lin Shen, Director, Department of Gastrointestinal Oncology and Phase I Clinical Trial Center at Peking University Cancer Hospital and Principal Investigator of the trial. “We are enthusiastic about the potential advantages this treatment brings to our patients.”
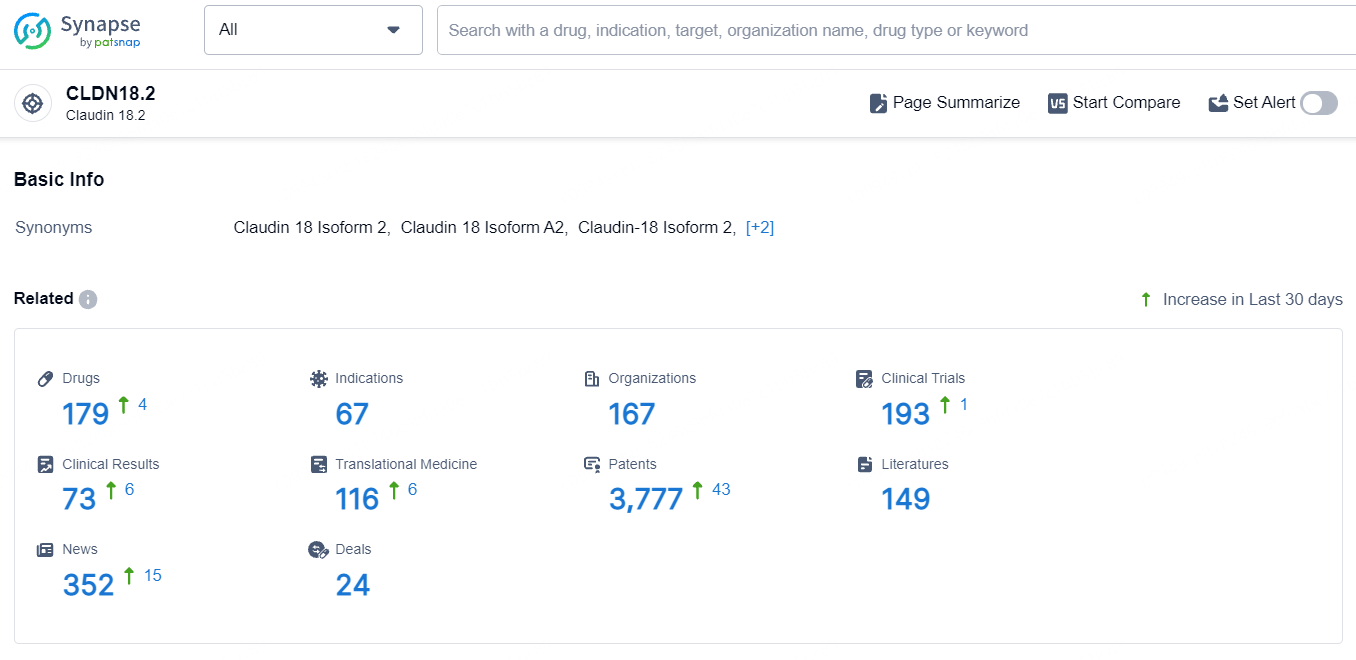
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of September 23, 2024, there are 179 investigational drugs for the CLDN18.2 target, including 67 indications, 167 R&D institutions involved, with related clinical trials reaching 193, and as many as 3777 patents.
Osemitamab is a monoclonal antibody drug developed by Suzhou Chuangsheng Pharmaceutical Group Co., Ltd. The drug targets CLDN18.2 and is designed to treat a range of diseases, including neoplasms, digestive system disorders, endocrinology, and metabolic diseases. Its active indications include CLDN18.2 positive gastroesophageal junction adenocarcinoma, stomach adenocarcinoma, bile duct neoplasms, advanced bile duct carcinoma, solid tumors, advanced cancer, advanced malignant solid neoplasm, gastroesophageal junction cancer, pancreatic cancer, pancreatic ductal adenocarcinoma, metastatic gastric adenocarcinoma, and metastatic gastroesophageal junction adenocarcinoma.