Promising Results from Avidity's Phase 1/2 FORTITUDE™ Trial of AOC 1020: Over 50% DUX4 Gene Reduction and Functional Improvements in FSHD Patients
Avidity Biosciences, Inc., a biopharmaceutical firm focused on developing a novel category of RNA therapeutics known as Antibody Oligonucleotide Conjugates, revealed promising initial data from the Phase 1/2 FORTITUDE™ study of AOC 1020. The results showed unprecedented and consistent reductions exceeding 50% in DUX4 regulated genes, along with indications of functional enhancement and positive safety and tolerability outcomes for individuals with facioscapulohumeral muscular dystrophy.
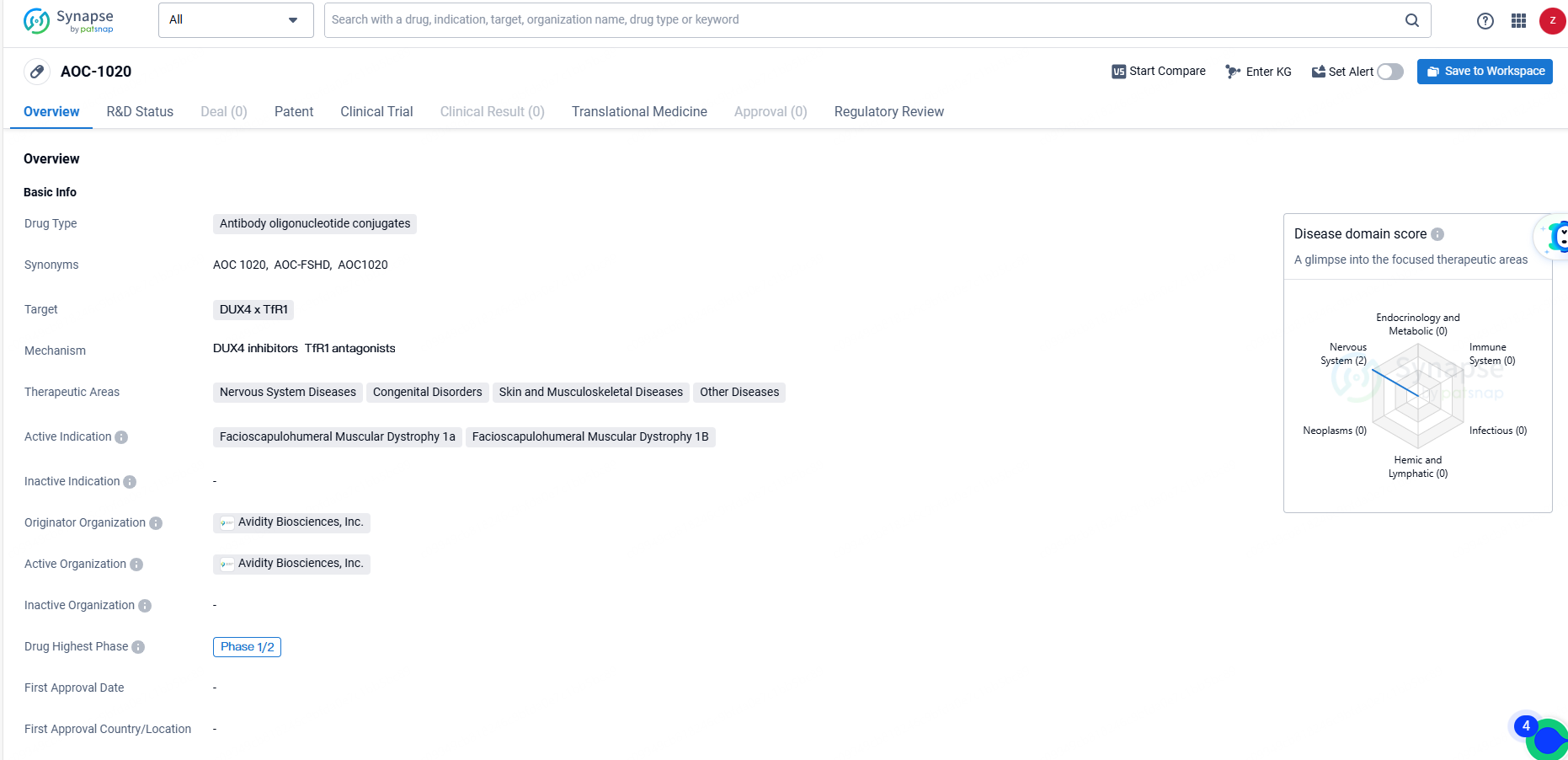
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Avidity intends to fast-track the start of registrational cohorts in its FORTITUDE™ trial. Additionally, Avidity has announced that the official international nonproprietary name for AOC 1020 will be delpacibart braxlosiran, abbreviated as del-brax.
Del-brax represents the initial investigative therapy aimed at addressing the root cause of FSHD, a condition resulting from abnormal expression of the double homeobox 4 (DUX4) gene. FSHD is a rare hereditary disorder characterized by relentless and progressive muscle function loss, significant pain, fatigue, and increasing disability. Currently, there are no therapies approved for FSHD treatment.
"As the first therapy to target DUX4, the data on del-brax is promising, showing consistent reductions in DUX4-regulated genes and indicating potential functional improvements in FSHD patients at the four-month mark. These preliminary findings support the potential of del-brax to alter the disease trajectory for those with FSHD," remarked Jeffrey M. Statland, M.D., Professor of Neurology at the University of Kansas Medical Center and an investigator in the FORTITUDE™ trial.
Del-brax (AOC 1020) is engineered to tackle the root cause of FSHD, which stems from the anomalous expression of the DUX4 gene. The aberrant DUX4 protein expression results in altered gene expression in muscle cells, correlating with progressive muscle function decline in FSHD patients.
The objective of del-brax is to diminish the expression of DUX4 mRNA and DUX4 protein in the muscles of those with FSHD. Del-brax comprises a specialized monoclonal antibody that binds to the transferrin receptor 1 (TfR1), conjugated with a siRNA targeting DUX4 mRNA. Preclinical trials demonstrated that a single intravenous dose of the murine analog of del-brax prevented muscular weakness, as evidenced by three functional tests: treadmill running, in vivo force measurement, and compound muscle action potential assessment.
Del-brax is currently undergoing Phase 1/2 development as a component of the FORTITUDE™ study in adults with FSHD. It has received Orphan designation from both the U.S. Food and Drug Administration and the European Medicines Agency, while the FDA has also granted del-brax Fast Track designation.
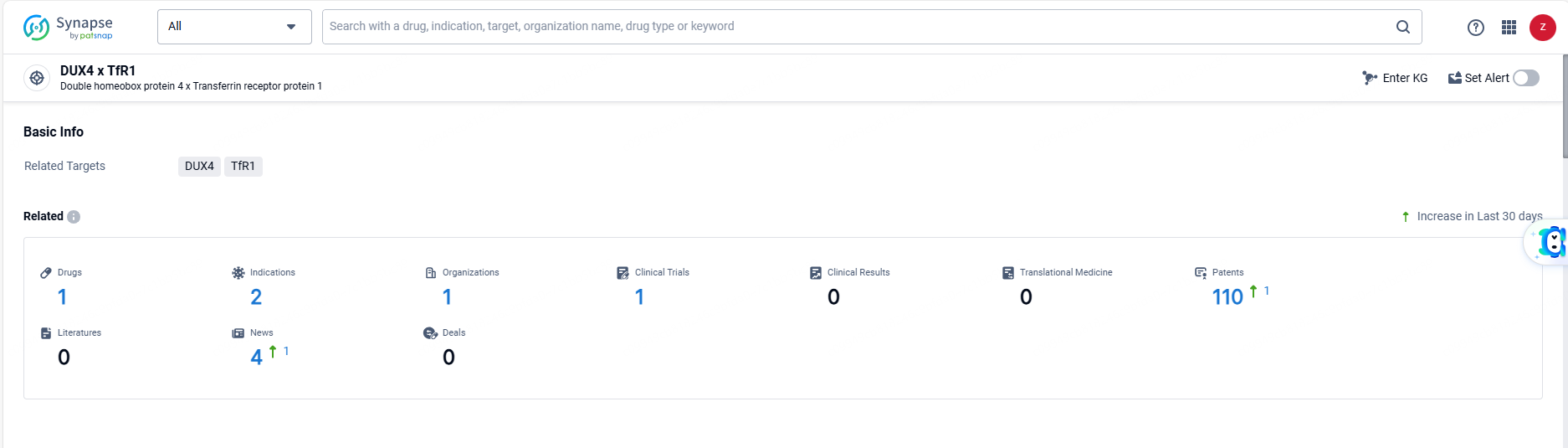
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 14, 2024, there are 1 investigational drugs for the DUX4 and TfR1 target, including 2 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 110 patents.
AOC-1020 is an antibody oligonucleotide conjugate developed by Avidity Biosciences, Inc. for the treatment of various diseases, with a focus on Facioscapulohumeral Muscular Dystrophy 1a and 1B. The drug has reached Phase 1/2 in its development and has been granted Fast Track and Orphan Drug designations, underscoring its potential to address unmet medical needs in rare and serious conditions.