Promising Safety Results from Neurogene's Phase 1/2 Study of NGN-401 Gene Therapy for Rett Syndrome
Neurogene Inc., a company operating at the clinical stage and dedicated to developing transformative genetic therapies for individuals and families grappling with rare neurological disorders, revealed preliminary data on safety and tolerability from their active Phase 1/2 clinical trial of gene therapy for Rett syndrome. The results indicate that NGN-401 was broadly tolerated across the three treated patients, who have been monitored for about nine, six, and three months after receiving their doses.
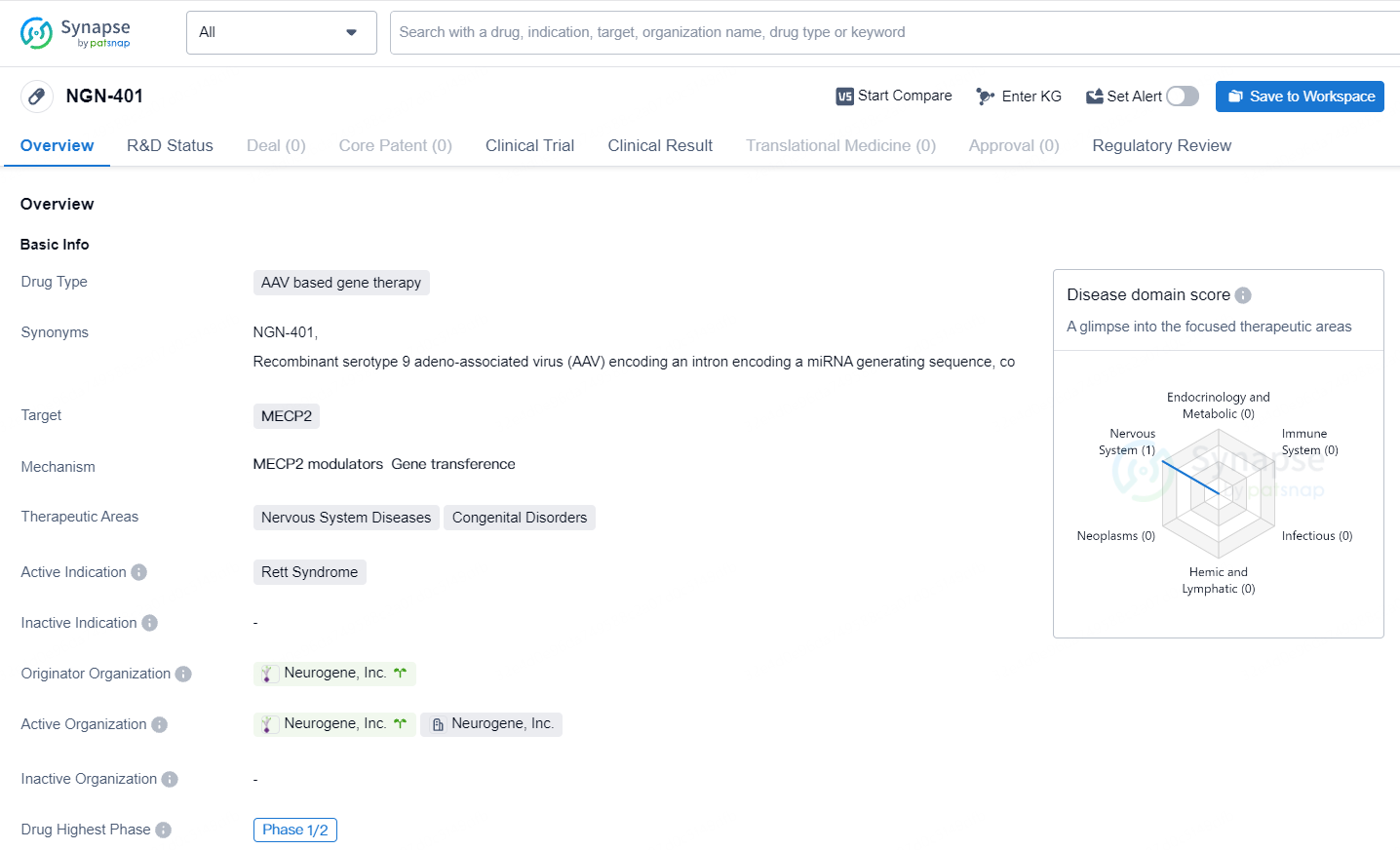
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Rachel McMinn, Ph.D., founder and CEO of Neurogene, explained that NGN-401 was developed with the purpose of addressing the shortcomings of traditional gene therapies for Rett syndrome. This innovation incorporates the proprietary EXACTTM technology for transgene regulation, which is believed to safely deliver beneficial protein levels specifically to critical regions in the brain and nervous system affected by the disease.
McMinn further noted, "The initial findings from NGN-401, shared at the ASGCT event, show a positive safety profile in the initial three children treated, one of whom possesses a mild variant that likely allows for some native MeCP2 protein activity. Importantly, none of these patients have exhibited any toxicity symptoms related to excessive protein expression. And we are on schedule to reveal preliminary efficacy outcomes from the initial patient group by late 2024."
The ongoing Phase 1/2 open-label trial aims to assess NGN-401's safety, tolerability, and initial effectiveness following a one-time intracerebroventricular infusion at two different dosage levels. Recruitment is currently active for female participants aged 4 to 10 years who have classic Rett Syndrome and exhibit a severity score ranging from 4 to 6 on the Clinical Global Impression-Severity scale, in both the lower-dosed first cohort and the higher-dosed second cohort.
The treatment has been generally well-received by all three participants thus far, with only mild or Grade 1 adverse events reported, which are typically short-lived or self-resolving. The most common side effects related to the adeno-associated virus used in the therapy include changes in lab values that do not cause symptoms.
Furthermore, there have been no reports of symptoms suggesting toxicity due to excessive MeCP2 protein levels for any of the patients, including the individual with the mild variation expected to maintain some residual MeCP2 activity. Additionally, there have been no serious adverse events linked to the treatment or the intracerebroventricular infusion procedure among these patients.
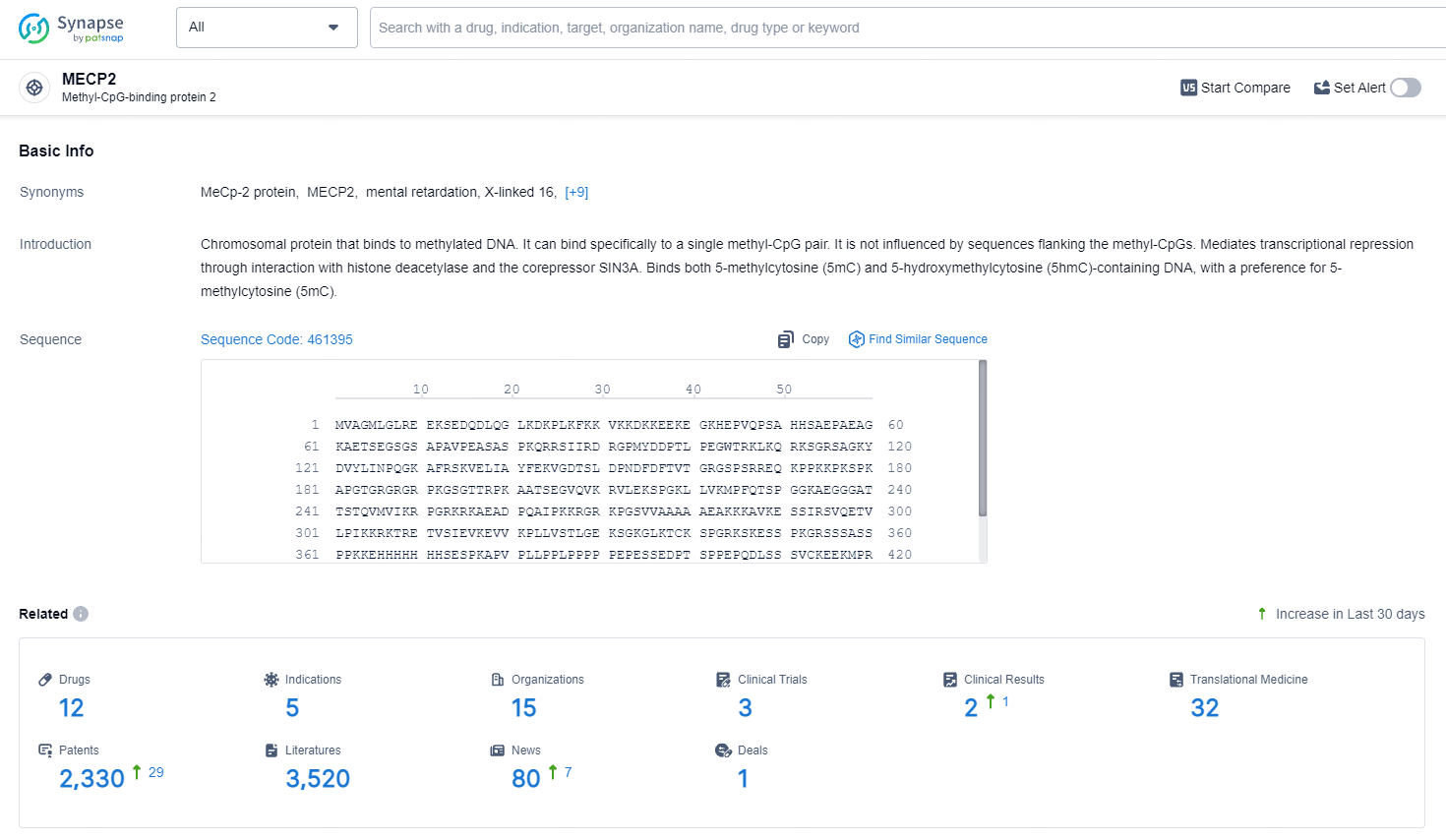
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 11, 2024, there are 12 investigational drugs for the MECP2 target, including 5 indications, 15 R&D institutions involved, with related clinical trials reaching 3, and as many as 2330 patents.
NGN-401 shows promise as a potential treatment for Rett Syndrome, a rare congenital disorder affecting the nervous system. Its AAV-based gene therapy approach and specific targeting of MECP2 make it a unique and innovative drug candidate. The fact that it has reached Phase 1/2 of clinical development indicates that it has already shown some positive results in early testing. The regulatory designations it has received further highlight the potential importance of NGN-401 in addressing the unmet medical needs of patients with Rett Syndrome.