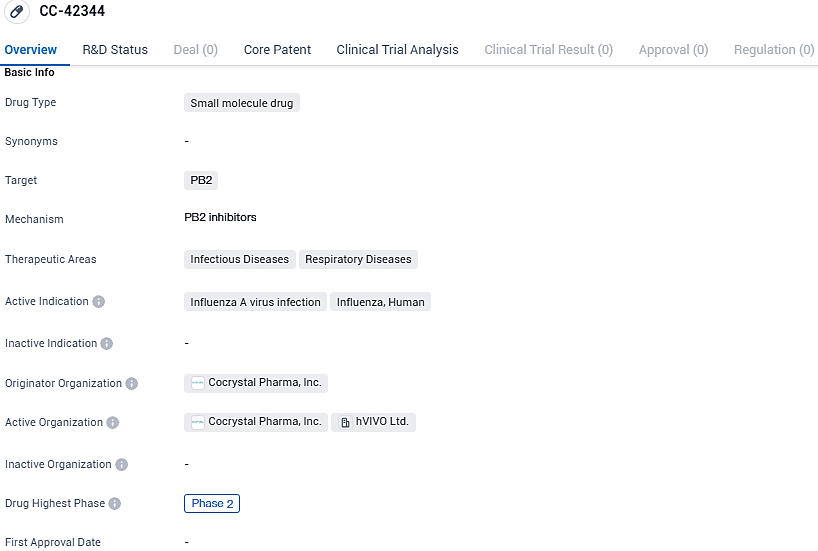
Cocrystal Pharma has begun enrolling the first participant in a Phase 2a trial to evaluate oral CC-42344's efficacy against pandemic and seasonal influenza A
Cocrystal Pharma, Inc. has reported that the initial participant has been enrolled in a Phase 2a human challenge trial for their new oral drug candidate, CC-42344, purposed for countering both pandemic and typical influenza A strains. This trial, which is designed as randomized, double-blinded, and featuring a placebo control, aims to assess the investigational antiviral's safety profile, its tolerability, and to monitor both viral response and clinical outcomes in individuals administered with the CC-42344 therapy by oral route.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
There's a pressing demand for the development of novel oral antiviral agents that combat both pandemic and regular seasonal flu strains, especially those capable of overcoming issues with antiviral resistance. Our company's unique structure-based drug-discovery platform led to the finding of CC-42344, geared at impeding the viral replication mechanism. "The insights obtained from this initial clinical proof-of-concept study will provide additional confirmation of the unique action mechanism of CC-42344," mentioned Dr. Sam Lee, who holds the positions of President and co-CEO at Cocrystal. "We anticipate sharing the primary findings from this clinical study in the year 2024."
James Martin, who shares co-CEO duties with Lee and also serves as Cocrystal's CFO, indicated his enthusiasm regarding the transformative potential of CC-42344 for treating what is globally one of the most prevalent viral diseases. He observed, "Existing antiviral medications for the flu often face problems with resistance from the virus. This reality amplifies the necessity for improved flu therapies that will benefit patients and, concurrently, lead to substantial financial efficiencies within worldwide healthcare."
Towards the end of 2022, Cocrystal announced promising findings related to safety and tolerability from the first stages of a Phase 1 trial that administered increasing doses to healthy volunteers in Australia. Preclinical studies have indicated the strong efficacy of CC-42344 against a variety of influenza A viruses, inclusive of those responsible for seasonal outbreaks and pandemics.
As of October 2023, Cocrystal was granted green light by the UK Medicines and Healthcare Products Regulatory Agency to commence a Phase 2a human challenge trial, examining CC-42344's potential as a remedial option for influenza A, in both pandemic and seasonal contexts.
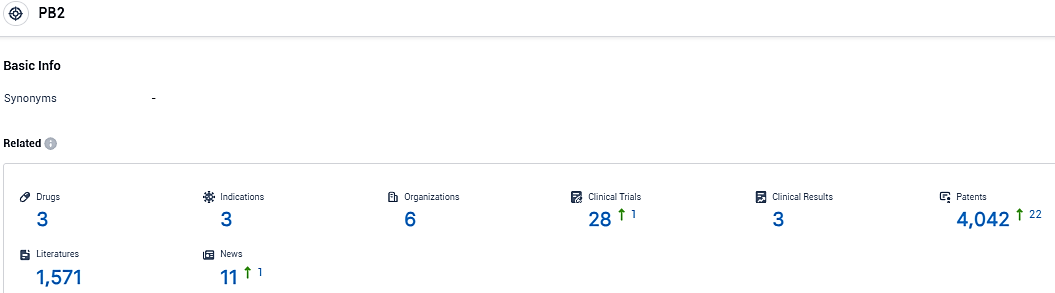
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 13, 2023, there are 3 investigational drugs for the PB2 target, including 3 indications, 6 R&D institutions involved, with related clinical trials reaching 28, and as many as 4042 patents.
CC-42344 targets PB2 and is indicated for the treatment of Influenza A virus infection and Influenza in humans. The drug has reached Phase 2 in global clinical trials, indicating promising results in terms of safety and efficacy. However, in China, CC-42344 is still in the preclinical phase, suggesting that further development and testing are required before it can progress to human trials in the Chinese market.