Ranok Therapeutics Expands RNK05047 Clinical Trials to China
Ranok Therapeutics, an emerging enterprise at the clinical phase in the biopharmaceutical industry, has unveiled the commencement of administering doses to participants in China as part of an early-phase clinical trial. This trial is for RNK05047, which represents the company's initial foray into the development of breakthrough protein degraders. These innovative therapeutics are designed to selectively dismantle specific proteins to combat various oncological conditions.
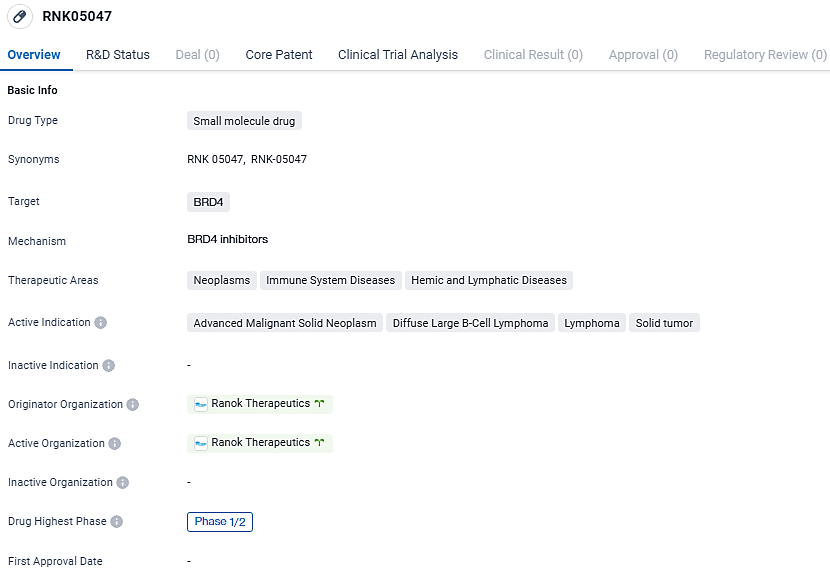
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The investigation, a counterpart to a currently active early-stage trial in the United States, aims to assess the harmlessness, tolerability, as well as the absorption and action mechanics of RNK05047 in Chinese individuals who are grappling with progressed malignancies or lymphatic system cancers. Ranok forecasts that early findings from the initial-stage inquiries in both the United States and China will be accessible by the closing of the year 2024.
"RNK05047, conceived via Ranok's exclusive CHAMP technique, represents the pioneering BRD4-specific protein degrader to be examined clinically within Chinese borders. This expands our existing clinical research of RNK05047 from the U.S. to include Asian patients, which we anticipate will yield significant understanding," stated Weiwen Ying, Ph.D., the founder and CEO of Ranok.
"BRD4 stands as an epigenetic focal point of significant interest in the realm of cancer therapy, applicable across diverse cancer manifestations," voiced Ning Li, M.D., Professor and VP at the National Cancer Center/Cancer Hospital, "The Cancer Hospital, trailblazing in both magnitude and reputation within China for cancer diagnosis and therapy, is thrilled to partake in this study with the prospect that RNK05047 will emerge as a valuable new treatment avenue for those suffering from cancer."
The Phase 1 clinical experiment, steered by Timothy Yap, M.D., Ph.D., a professor specializing in Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center, is meticulously crafted to affirm the initial concept utilizing SNV1521 through an oral, single-agent dose advancement and early effectiveness enlargement study. PARP inhibition is medically acknowledged for treating cancers fueled by homologous recombination insufficiency.
Nonetheless, the tolerability profile of the original class of PARP1 inhibitors constrains the overall potential of this category, especially when utilized in tandem with chemotherapeutic agents or cutting-edge compounds. Studies on preclinical test subjects have revealed that focused targeting of PARP1 has the potential to magnify the therapeutic success and harmlessness of these inhibitors.
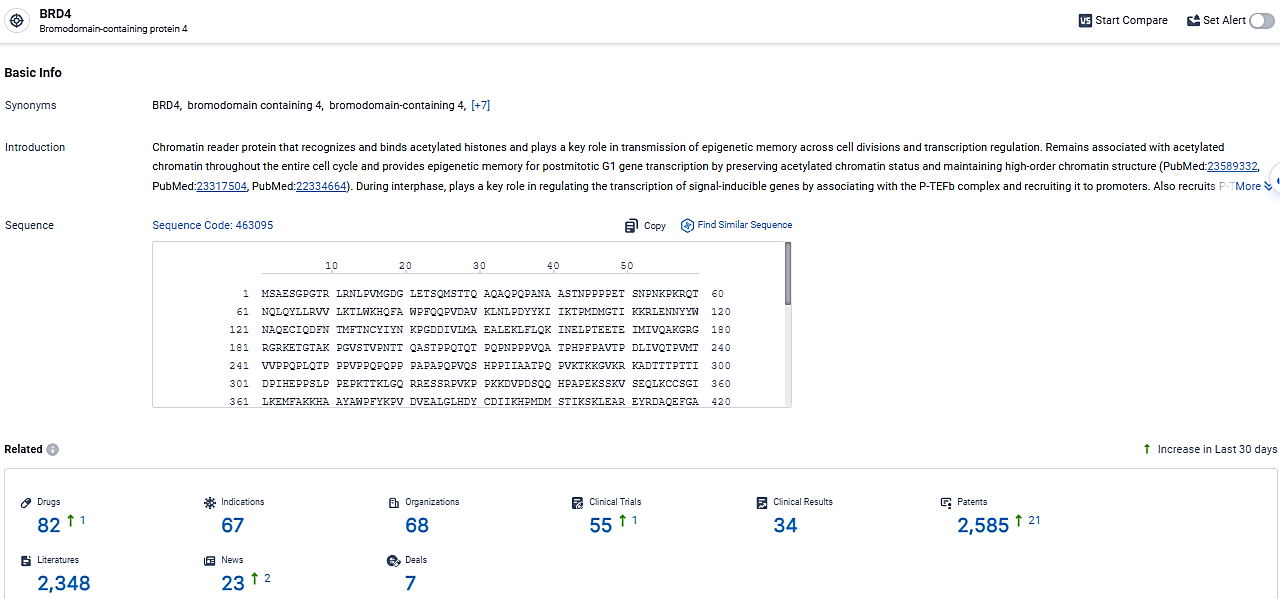
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 12, 2024, there are 82 investigational drugs for the BRD4 target, including 67 indications, 68 R&D institutions involved, with related clinical trials reaching 55, and as many as 2585 patents.
RNK05047 is a first-in-class, small-molecule, tumor- and BRD4-selective protein degrader that was discovered and developed using Ranok’s proprietary approach to targeted protein degradation, CHAMP. The active indications for RNK05047 include advanced malignant solid neoplasm, diffuse large B-cell lymphoma, lymphoma, and solid tumors. Further research and development will be needed to determine the safety and efficacy of this drug in treating these conditions.