MoonLake's Sonelokimab Shows Promising Six-Month Results for Psoriatic Arthritis Treatment at R&D Event
MoonLake Immunotherapeutics has disclosed that ongoing therapy using the proprietary Nanobody® (sonelokimab), has yielded notable advancements in every primary endpoint in the 24-week findings from the ARGO study, focusing on psoriatic arthritis. Further substantial updates in research and development will be comprehensively conveyed and examined during the firm's Research and Development Day, scheduled for this present day.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In the ARGO study which included a cohort of 207 individuals experiencing symptomatic PsA, it was observed that the principal measure of success, denoted as the American College of Rheumatology 50, continued its upward trajectory from the 12-week mark, reaching a threshold above 60% by the 24th week. Moreover, a sizeable fraction, roughly 40%, attained the more demanding ACR70 benchmark by the same juncture. Treatment with sonelokimab by the 24-week period led to significant strides in skin clearance, with upwards of 80% and 60% of the participants achieving a Psoriasis Area Severity Index of 90 and 100, respectively.
Therapeutic efficacy was comparable across all administered dosages of sonelokimab. These outcomes outstripped those observed in the adalimumab-receiving control group of the study, and also fared better when contrasted indirectly with competitor treatments using adalimumab as a common referential yardstick.
Dr. Kristian Reich, holding dual roles as the Founder and Chief Scientific Officer at MoonLake, remarked on the implications of the 24-week data from the ARGO study, noting that ongoing sonelokimab intervention led to marked enhancements in all primary effectiveness measures. This underscores the potential benefits in leveraging a more diminutive biopharmaceutical entity with albumin-binding properties, which can obstruct IL-17F along with IL-17A, in addressing deep-seated inflammatory conditions.
Building on preliminary findings released in November 2023, these 24-week insights are poised to be featured in an upcoming publication within a scientifically rigorous periodical. As it stands, regulatory approval for sonelokimab for any medical use has not been obtained.
Professor Kenneth B. Gordon, who heads the Dermatology department at the Medical College of Wisconsin, remarked on the advancement of Phase 3 trials. He expressed optimism for sonelokimab, highlighting the anticipation of those affected by HS for novel treatment avenues that offer enduring relief, positing sonelokimab as a potentially effective therapeutic candidate for this patient demographic.
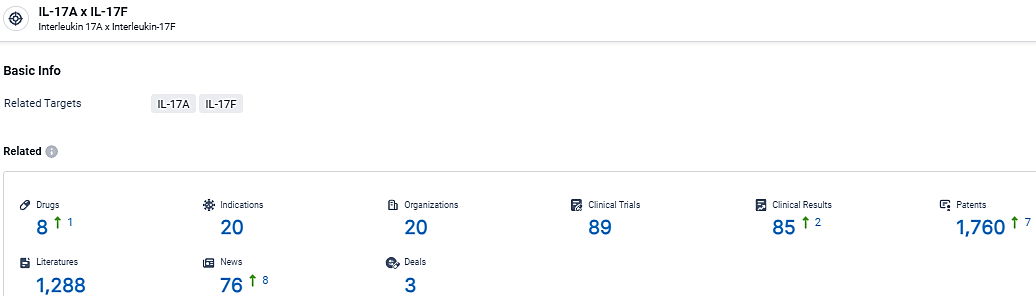
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 12, 2024, there are 8 investigational drugs for the IL-17A x IL-17F target, including 20 indications, 20 R&D institutions involved, with related clinical trials reaching 89, and as many as 1760 patents.
With two domains, sonelokimab selectively binds with high affinity to IL-17A and IL-17F, thereby inhibiting the IL-17A/A, IL-17A/F, and IL-17F/F dimers. A third central domain binds to human albumin, facilitating further enrichment of sonelokimab at sites of inflammatory edema. Currently in Phase 2, Sonelokimab has the potential to provide a novel therapeutic option for patients suffering from these conditions.