REGENXBIO Announces Positive Phase II Results for ABBV-RGX-314 in Wet AMD at AAO 2024
REGENXBIO Inc. (Nasdaq: RGNX) has shared encouraging results from the Phase II sub-study focusing on fellow eyes, which assesses the subretinal administration of ABBV-RGX-314 in individuals suffering from bilateral wet age-related macular degeneration (wet AMD). These findings were presented at the American Academy of Ophthalmology (AAO) conference by Arshad Khanani, M.D., M.A., FASRS, the Director of Clinical Research at Sierra Eye Associates in Reno, NV.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The findings shared at the AAO regarding the Phase II sub-study represent the initial assessment of a gene therapy for fellow eyes in the context of wet AMD. They indicate that ABBV-RGX-314 may offer a therapeutic option for individuals with bilateral conditions, further enhancing the substantial evidence supporting ABBV-RGX-314’s potential to reshape treatment approaches for patients suffering from wet AMD," stated Curran Simpson, President and CEO of REGENXBIO. "With an increased number of treated individuals and the most extensive duration data of any gene therapy initiative for wet AMD, REGENXBIO, alongside our collaborator AbbVie, is strategically positioned to introduce the first gene therapy into the market, aiming to preserve the long-term vision for millions of people worldwide affected by wet AMD."
"Most of our wet AMD patients eventually develop bilateral disease, which imposes a considerable treatment burden due to the necessity of frequent, lifelong injections in both eyes. This situation often results in less than optimal vision outcomes in real-world scenarios with the existing standard treatments,” remarked Dr. Khanani. “The data for fellow eye administration of ABBV-RGX-314 marks a significant advancement in the area of gene therapies for prevalent retinal diseases, representing the first bilateral intervention for patients with wet AMD. These findings, together with the enduring treatment effects observed over four years in long-term follow-ups, underscore the potential of ABBV-RGX-314 as an effective one-time therapeutic solution for patients with wet AMD.”
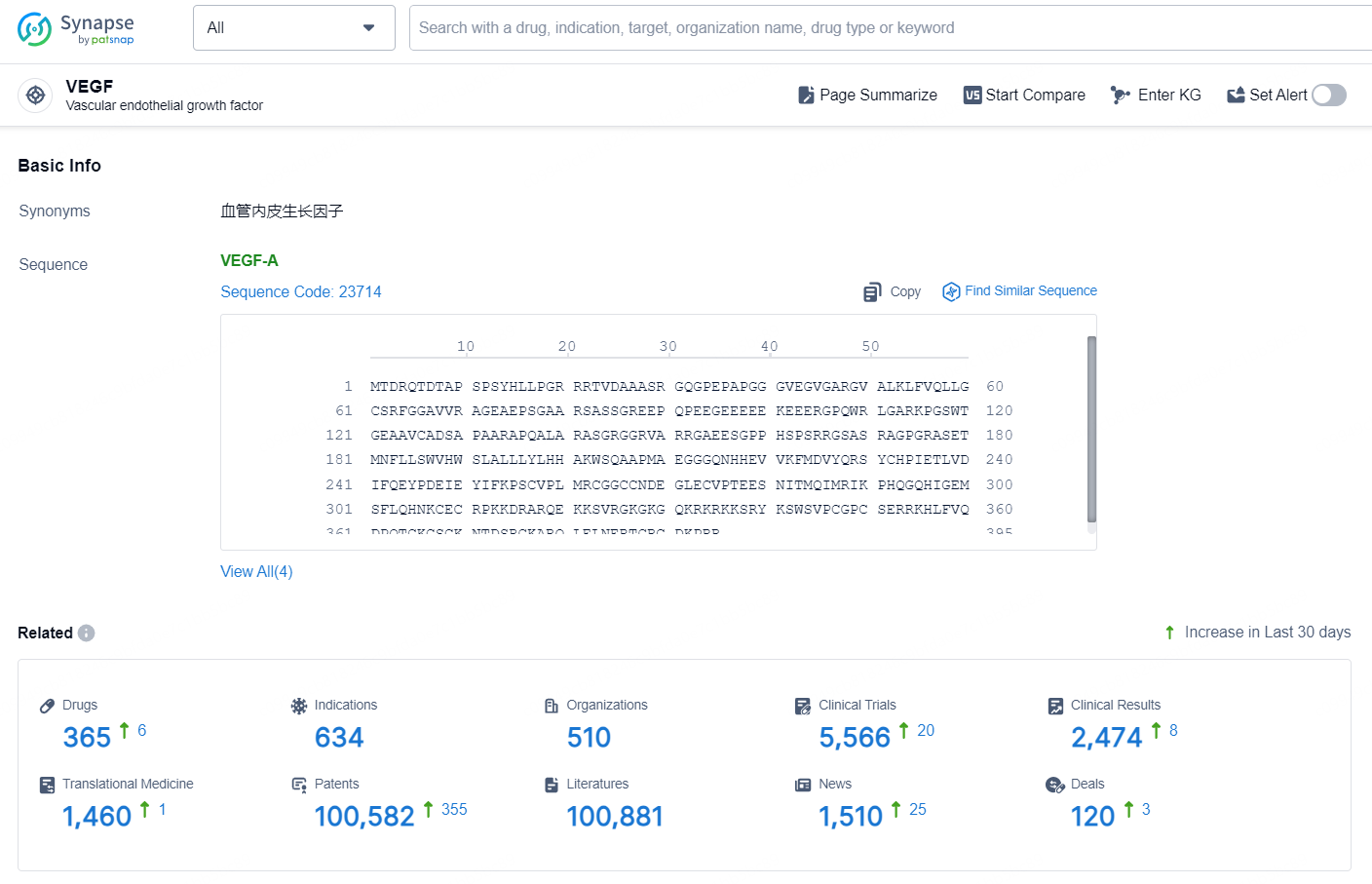
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 365 investigational drugs for the VEGF target, including 634 indications, 510 R&D institutions involved, with related clinical trials reaching 5566, and as many as 100582 patents.
RGX-314 is an AAV (adeno-associated virus) based gene therapy drug developed by RegenxBio, Inc. The drug targets VEGF (vascular endothelial growth factor) and is being developed for the treatment of various therapeutic areas, including Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, Eye Diseases, Other Diseases, and Cardiovascular Diseases.