REGENXBIO Reveals Clinical Information from Phase I/II Study of RGX-202
REGENXBIO Inc. disclosed further provisional safety information and preliminary effectiveness results from the phase I/II AFFINITY DUCHENNE™ study of RGX-202 aimed at treating Duchenne Muscular Dystrophy. The findings were presented at the 28th yearly International Gathering of the World Muscle Society.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug. 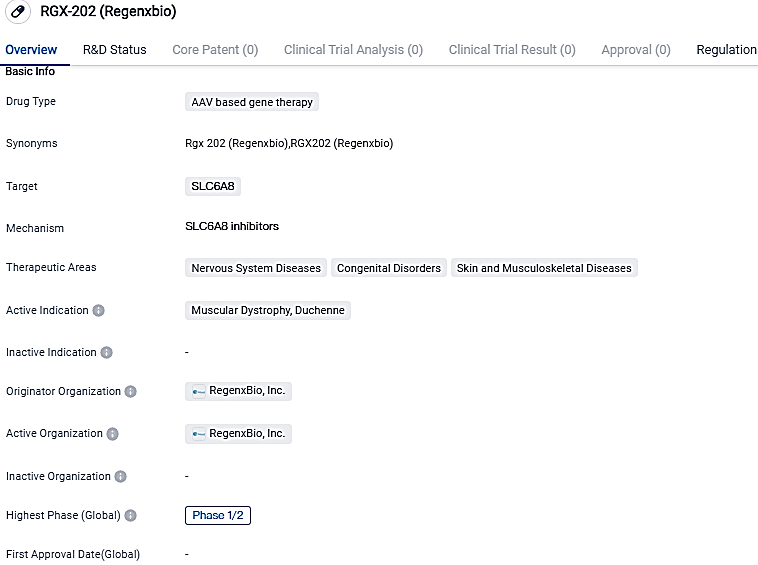
"Duchenne represents a rare form of a degenerative ailment that progressively weakens muscles due to the lack of a functional dystrophin protein. This leads to mobility loss as well as decreasing pulmonary and heart functions," stated Olivier Danos, Ph.D., the Principal Scientific Officer at REGENXBIO. "RGX-202's distinctive design, incorporating the C-Terminal domain, could be a game-changer for patients. We view the preliminary safety and effectiveness results with optimism."
RGX-202 is an investigational one-time AAV treatment currently under investigation for Duchenne. It employs the NAV® AAV8 vector to transmit a transgene for a novel microdystrophin, which involves the C-Terminal domain's operational components and a muscle-specific promoter. This configuration supports targeted therapy to enhance muscle's resistance to damage linked with Duchenne.
Preliminary biomarker data derived from a trio of monthly trial assessments completed by two patients exhibited promising growth in RGX-202 microdystrophin expression. These results were derived from bicep muscle biopsies conducted three months after a one-time provision of RGX-202. Additionally, the presence of RGX-202 microdystrophin was confirmed through immunofluorescence staining in muscle tissue after three months, with RGX-202 microdystrophin protein being confined to the sarcolemma.
The levels of RGX-202 microdystrophin were calculated employing a precise and mechanized western blot approach and validated using a specific liquid chromatography-mass spectrometry procedure.
"I am optimistic about the primary outcomes that indicate RGX-202 microdystrophin to be well-tolerated while enabling powerful microdystrophin expression in the muscle tissue. These form key premature discoveries," stated Aravindhan Veerapandiyan, M.D., Pediatrics Neuromuscular Neurology Specialist, Arkansas Children's Hospital. "I am acutely aware of the ongoing necessity for fresh treatment paradigms with the potential to modify the disease's progression for these young boys."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
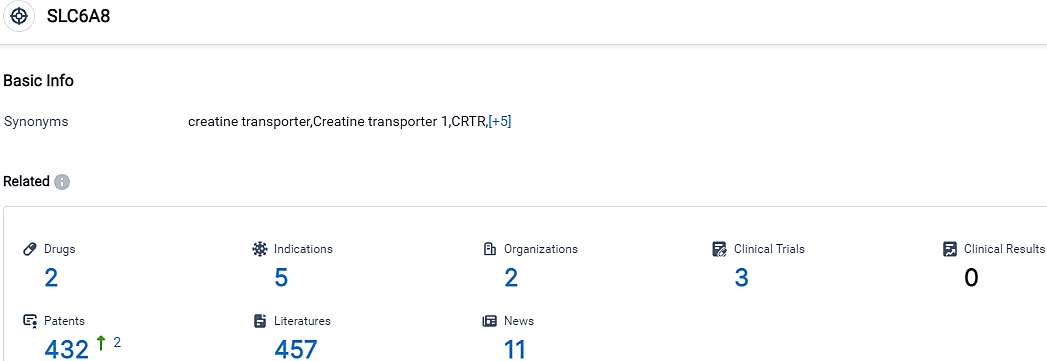
According to the data provided by the Synapse Database, As of October 14, 2023, there are 2 investigational drugs for the SLC6A8 target, including 5 indications, 2 R&D institutions involved, with related clinical trials reaching 3,and as many as 432 patents.
RGX-202 is designed to deliver a transgene for a novel microdystrophin that includes the functional elements of the C-Terminal domain found in naturally occurring dystrophin. RGX-202 has been granted Fast Track and Orphan Drug designations, highlighting its potential to address the urgent medical needs of patients with this rare and debilitating condition.