RemeGen Presents RC48 (Disitamab Vedotin) Cervical Cancer Study at 2024 ESGO
RemeGen Co. Ltd., which is actively commercializing its products, delivered a spoken summary outlining a provisional review of their unique research on Disitamab Vedotin (RC48). This experimental ADC (antibody-drug conjugate) aims at widespread solid neoplasms associated with substantial clinical demand gaps. This update took place during the European Congress on Gynaecological Oncology event in Barcelona, spanning from the 7th to the 10th of March, 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 At present, a Phase II, non-blinded, multi-institutional umbrella trial is in progress to ascertain the efficacy and safety profile of RC48 as a sole treatment in cases of gynecological cancers that exhibit HER2 positivity. The study segment focusing on cervical cancer encompasses individuals enduring recurrent or metastatic forms of the disease who have shown disease progression following a minimum of one prior line of anti-cancer treatment and present with a HER2 IHC level of 1+ or higher.
At present, a Phase II, non-blinded, multi-institutional umbrella trial is in progress to ascertain the efficacy and safety profile of RC48 as a sole treatment in cases of gynecological cancers that exhibit HER2 positivity. The study segment focusing on cervical cancer encompasses individuals enduring recurrent or metastatic forms of the disease who have shown disease progression following a minimum of one prior line of anti-cancer treatment and present with a HER2 IHC level of 1+ or higher.
RC48 is being administered as the only treatment at a concentration of 2 mg/kg on a bi-weekly schedule. The chief measure of success for the trial is the rate of objective responses as determined by an Independent Review Committee, and this is supported by additional outcomes such as the response rate assessed by the Investigating team, response longevity, rate of disease control, time to disease progression, overall patient survival, and the tolerability of the treatment.
Dr. Jianmin Fang, the chief executive of RemeGen, expressed his enthusiasm regarding the forthcoming oral elucidation of the company's unique ADC, Disitamab Vedotin, at ESGO 2024. He emphasized that this reflects RemeGen's pledge to persistent innovation and progress in combating cervical cancer, and they firmly anticipate that RC48's findings will offer a novel ray of hope to those affected by cervical cancer.
Cervical cancer ranks as a prevalent cancer type within the field of gynecology. The 2022 cancer report from China indicated that patients with advanced stages of recurrent or metastatic cervical cancer have a five-year progression-free survival rate of merely around 15%. For individuals with recurrent or metastatic forms of cervical cancer, the therapeutic avenues are currently restricted to immunotherapeutic and chemotherapeutic approaches, highlighting a substantial gap in clinical care needs.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
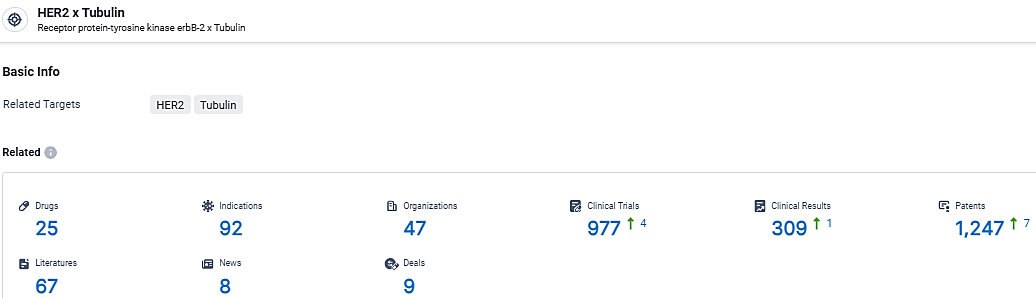
According to the data provided by the Synapse Database, As of March 12, 2024, there are 25 investigational drugs for the HER2 and Tubulin target, including 92 indications, 47 R&D institutions involved, with related clinical trials reaching 977, and as many as 1247 patents.
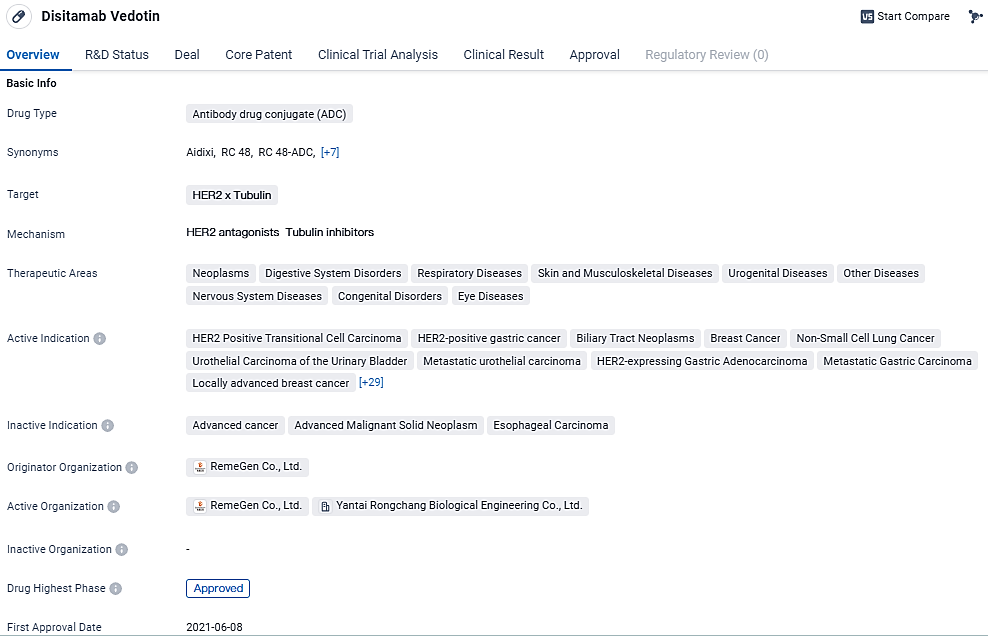
Disitamab Vedotin is an approved antibody drug conjugate that targets HER2 and Tubulin. It has shown efficacy in treating a wide range of cancers and has received approvals in China and globally. The drug's approval offers hope for patients with HER2-positive cancers and expands the treatment options available in the field of biomedicine.