Repare Therapeutics Begins Phase 1 Trial Dosing with RP-3467, a Polθ ATPase Inhibitor
Repare Therapeutics Inc. (Nasdaq: RPTX), a leading clinical-stage precision oncology company, announced the first patient has been dosed in the Company’s Phase 1 (POLAR) clinical trial evaluating RP-3467, a Polθ ATPase inhibitor, alone and in combination with the poly-ADP ribose polymerase (PARP) inhibitor, olaparib. RP-3467 is Repare’s fourth clinical program.
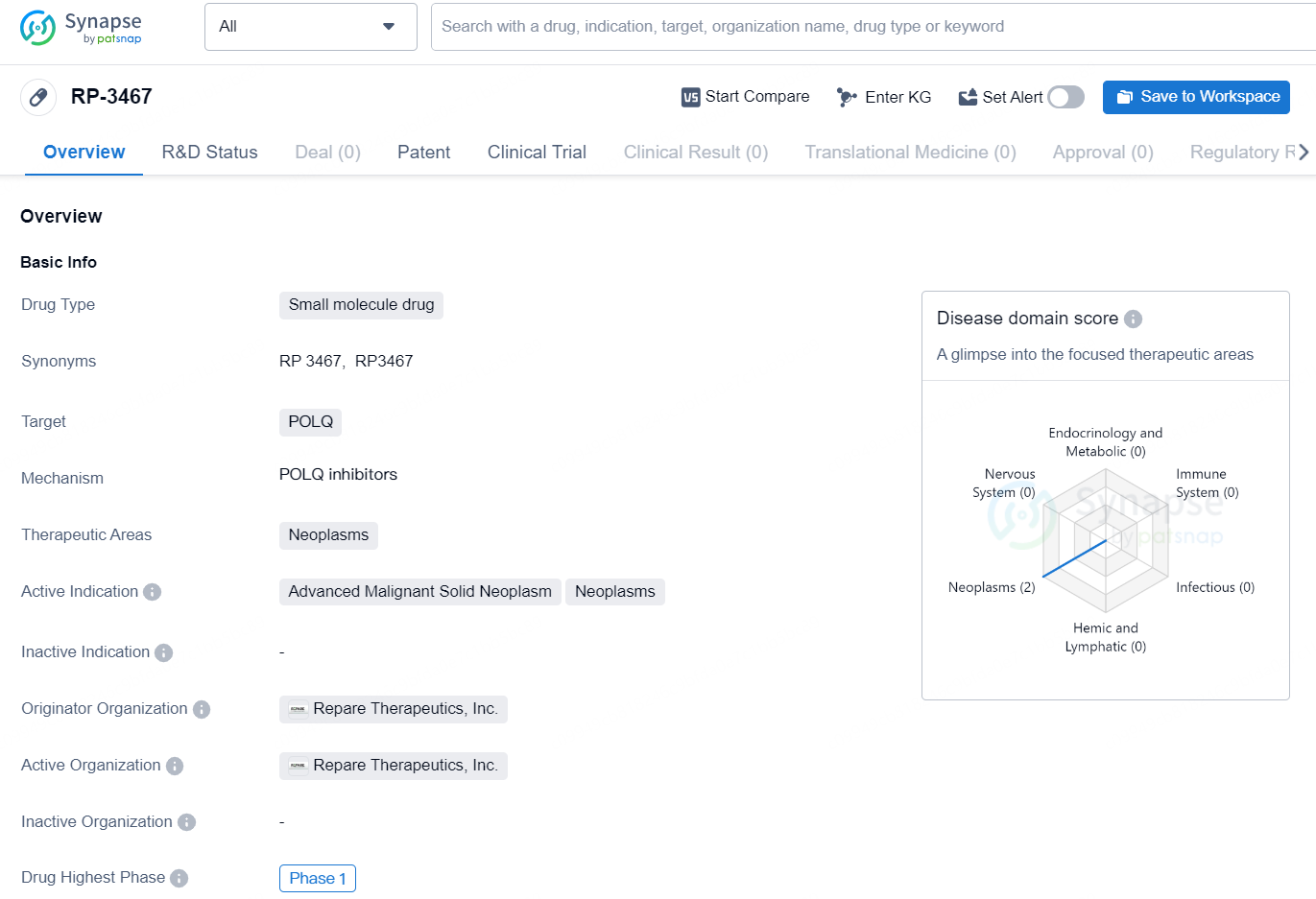
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
RP-3467, which shows promise as a leading Polθ ATPase inhibitor, has produced highly persuasive preclinical findings, including complete and lasting tumor regressions when paired with olaparib, a foremost PARP inhibitor, without causing additional toxic effects. According to Maria Koehler, MD, PhD, Executive Vice President and Chief Medical Officer of Repare, "This combination aims to significantly enhance patient outcomes by addressing the challenge of PARP inhibitor resistance, which represents a crucial area of unmet medical need." Furthermore, the data previously shared by Repare indicates that RP-3467 has the potential to not only enhance effectiveness but also reduce toxicity when combined with radioligand therapy and antibody-drug conjugates (ADCs). We are eager to investigate these possibilities further.
The POLAR clinical trial (NCT06560632) is a multicenter, open-label, dose-escalation Phase 1 study aimed at evaluating the safety, pharmacokinetics, pharmacodynamics, and preliminary clinical efficacy of RP-3467, either alone or together with olaparib, in adults with molecularly defined advanced solid tumors. The trial intends to recruit around 52 participants diagnosed with locally advanced or metastatic epithelial ovarian cancer, metastatic breast cancer, metastatic castration-resistant prostate cancer, or pancreatic adenocarcinoma. The primary goals of this study include determining the safety and tolerability of RP-3467 by itself and in conjunction with olaparib, as well as establishing a preliminary recommended Phase 2 dosage of RP-3467 when used alongside olaparib.
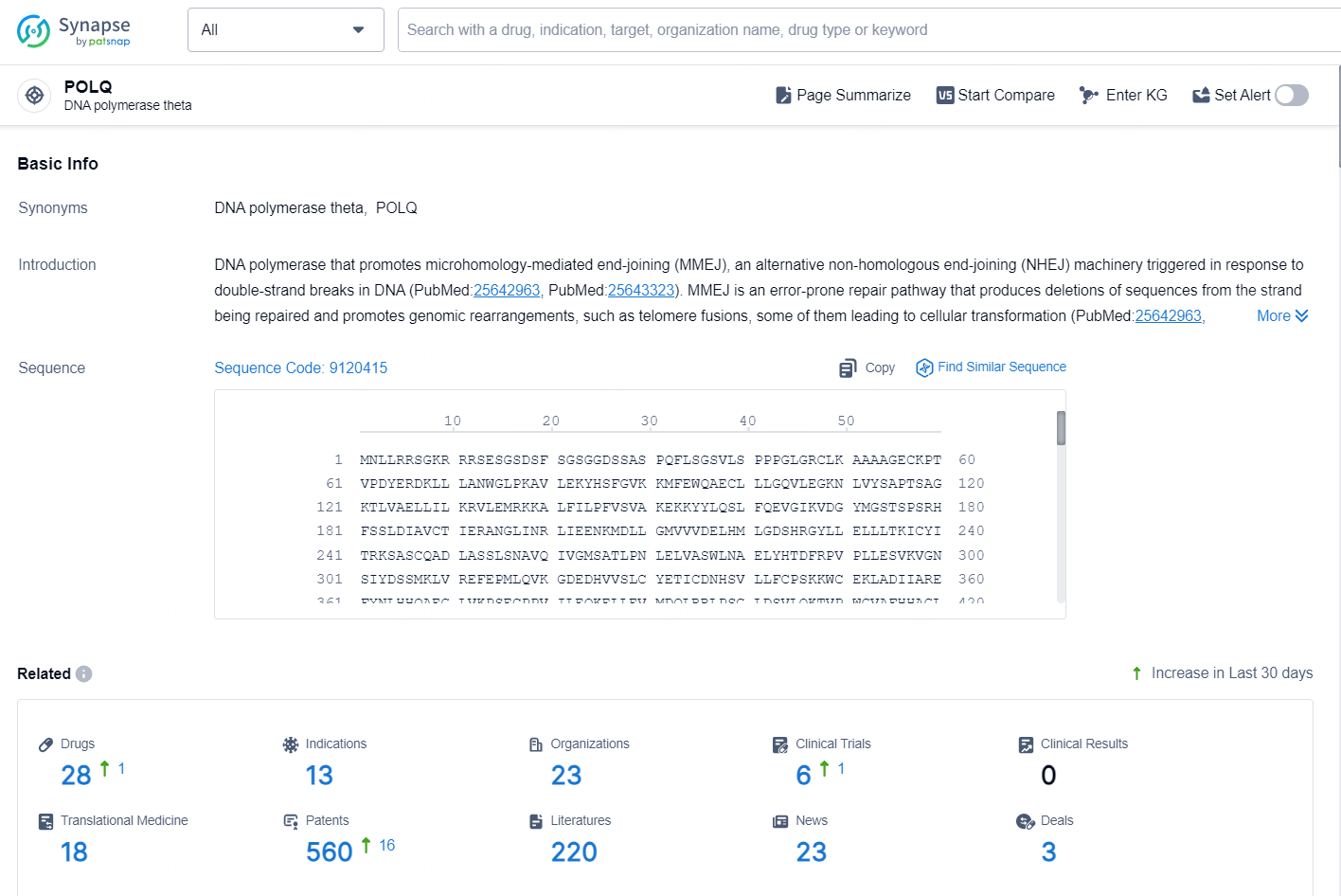
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 15, 2024, there are 28 investigational drugs for the POLQ targets, including 13 indications, 23 R&D institutions involved, with related clinical trials reaching 6, and as many as 560 patents.
RP-3467 is a small molecule drug developed by Repare Therapeutics, Inc. The drug targets the POLQ gene and is intended for the treatment of neoplasms, particularly advanced malignant solid neoplasms. Currently, RP-3467 is in the highest phase of development globally, which is Phase 1.