Results of Phase III Study of Enspryng in Patients with Generalized Myasthenia Gravis
Chugai Pharmaceutical Co., Ltd. has disclosed outcomes from the pivotal Phase III LUMINESCE trial involving their developed drug, Enspryng, known by the scientific name satralizumab (genetical recombination). This drug is currently under evaluation as a potential therapeutic option for generalized myasthenia gravis. Data from the primary outcome measure achieved statistical significance.
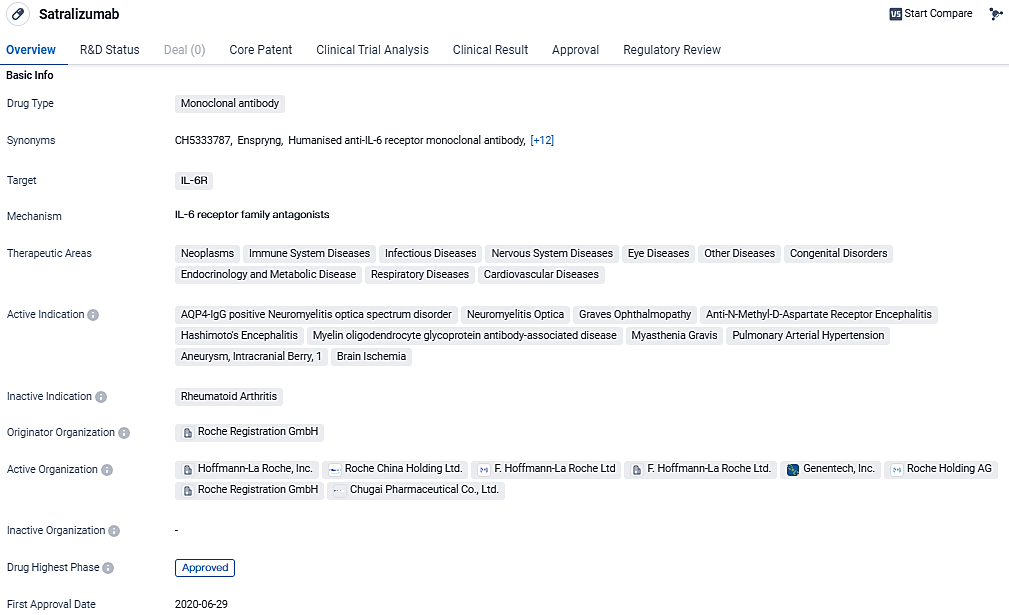
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The outcomes fell short of our anticipated level of therapeutic efficacy. Enspryng demonstrated a favorable level of tolerability in patients with gMG. Its safety profile aligned with its initial usage for neuromyelitis optica spectrum disorder. We will unveil comprehensive findings at the premiere American Academy of Neurology Annual Meeting in an oral presentation under the Emerging Science category on April 15 in Denver, Colorado.
The implications of these study findings for gMG does not alter Enspryng's established use in NMOSD, where it has gained approval. Together, Chugai and Roche endeavor to expand Enspryng’s use into further neurological disorders characterized by autoimmune and inflammatory responses, which could benefit from the suppression of IL-6 signaling. This includes conditions such as autoimmune encephalitis, myelin oligodendrocyte glycoprotein-associated disorder, and thyroid eye disease.
Developed by Chugai, Enspryng is a humanized anti-IL-6 receptor monoclonal antibody with pH-dependent binding capability, aimed at targeting IL-6. It has the distinction of being the inaugural therapy crafted using Chugai's unique Recycling Antibody technology. This new method enhances the duration of IL-6 suppression and allows for dosing via subcutaneous injection every four weeks, a marked improvement over traditional technologies.
Enspryng stands as the sole IL-6 inhibiting therapy that has received authorization in 89 nations for the treatment of aquaporin-4 immunoglobulin G-seropositive neuromyelitis optica spectrum disorder, with ongoing reviews by health authorities of its applications in additional countries.
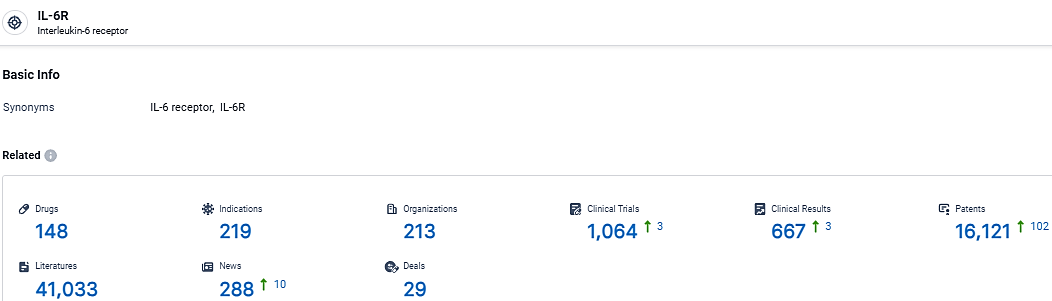
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 25 2024, there are 148 investigational drugs for the IL-6R target, including 219 indications, 213 R&D institutions involved, with related clinical trials reaching 1064, and as many as 16121 patents.
Satralizumab is a monoclonal antibody drug that targets IL-6R and has been approved for various therapeutic areas. Its active indications cover a wide range of diseases, including neurological, ophthalmic, and cardiovascular conditions. The regulatory designations further emphasize the potential of Satralizumab to address critical medical needs and accelerate its availability to patients.