Review and Prospects of CYP1A2 Inhibitors
Recombinant Cytochrome P450 1A2 (CYP1A2) belongs to the CYP1A subfamily of the CYP1 family and is encoded by the CYP1A2 gene. CYP1A2 is primarily located in the liver, accounting for about 13% of the total CYP450 enzymes in the liver, and is also expressed in the intestines, brain, and lungs. CYP1A2 participates in the metabolism of more than 20 drugs, including caffeine, warfarin, theophylline, lidocaine, mexiletine, verapamil, nitrendipine, and tacrine. Among them, caffeine, phenacetin and theophylline are often used as probe substrates to assess the in vivo activity of CYP1A2. The respective metabolic pathways of these three substrates include caffeine N3 demethylation, phenacetin O-demethylation and theophylline N-demethylation. In addition, CYP1A2 plays a significant role in the metabolism of some endogenous substances (steroids) and toxins, and certain precarcinogens (such as aflatoxins, nitrosamines). The enzymatic activity of CYP1A2 varies greatly among individuals and may be influenced by factors such as smoking, medication, genes, and other factors.
The secondary structure of CYP1A2 is dominated by alpha helices, consisting of 1 to 12 alpha helices (represented by the letters A to L), and also includes 4 beta sheets (represented by the numbers 1 to 4). All these secondary structures will further fold into the standard and unique regions of CYP450 enzyme structures within CYP1A2. The standard regions mainly include the regions linked to the heme co-factor and the regions connected to the electron-supplying cytochrome P450 reductase and Cytochrome b5 reductase. The unique regions refer to the narrow and flat active center unique to CYP1A2. As the active center of CYP1A2 is located inside the protein, some molecules involved in the CYP1A2 catalytic reaction, such as water molecules, oxygen molecules, and substrate molecules, must first pass through the substrate channel connecting the protein surface and the active center before entering the enzyme catabolic center. After the reaction, the resultant products may also be expelled outside the active center through the molecular channel.
The expression of CYP1A2 in the liver is regulated by the AhR and other metabolic pathways. After binding and nuclear translocation, transactivation mediated by AhR induces CYP1A2. AhR is a ligand-activated transcription factor, belonging to the basic helix-loop-helix (bHLH) Per-ARNT-Sim homology (PAS) family of transcription factors. The 90kDa heat shock protein (Hsp), prostaglandin E synthase 3 (p23), and the 43kDa Hepatitis B X-associated protein 2 (XAP2, also known as AhR1 interacting protein) are involved in the regulation of AhR. AHR exists as a cytosolic complex binding with HSPs, p23, and XAP2. Upon ligand binding, Ah receptor nuclear translocator (Arnt) replaces its associated molecules (Hsps, p23, and XAP2) to form a heterodimer, and 90kDa HSPs are released as AhR is translocated into the nucleus. This heterodimer interacts with the 5GCGTG-3DNA sequence to regulate the expression of the CYP1A2 gene. The AhR-mediated CYP1A1/1A2 pathway helps maintain dynamic equilibrium in the cellular environment by enhancing the metabolism of substrates.
In addition, the regulation of CYP1A2 may involve other mechanisms, including degradation of CYP1A2 mRNA and protein, cis and trans activation of transcription factors, alternative splicing, RNA stability, microRNAs, and epigenetics (such as DNA methylation).
CYP1A2 Competitive Landscape
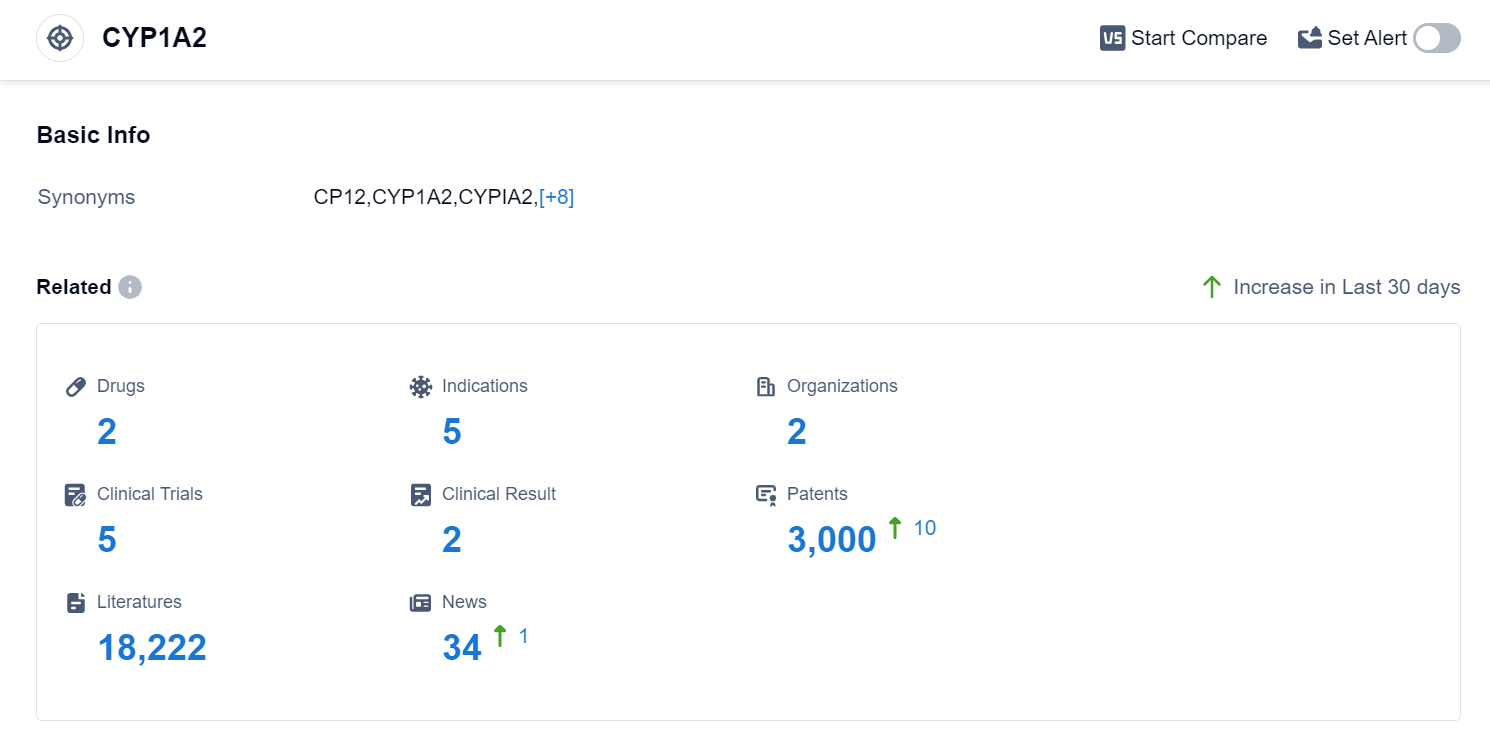
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 2 CYP1A2 drugs worldwide, from 2 organizations, covering 5 indications, and conducting 5 clinical trials..
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis of the provided data, the current competitive landscape for the target CYP1A2 indicates that Merck & Co., Inc. and Takeda Pharmaceutical Co., Ltd. are the leading companies in terms of R&D efforts. However, both companies are currently in the inactive phase of development.
In terms of geographical distribution, Japan, the United States, and Australia are actively involved in the development of drugs targeting CYP1A2.
Overall, the target CYP1A2 is still in the early stages of development, with further research and development required to advance the pipeline and potentially bring new drugs to market.