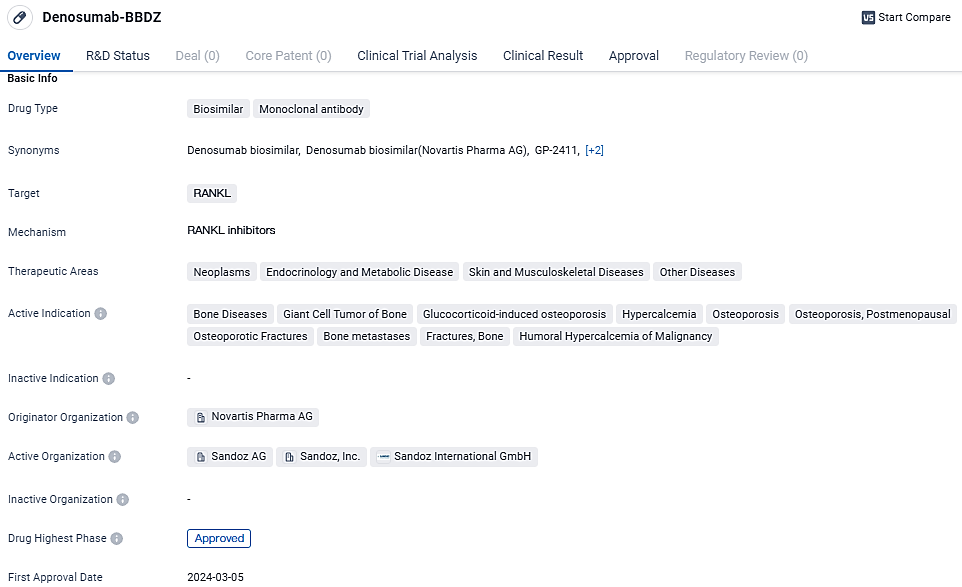
Sandoz Obtains US Health Regulator's Green Light for Unique Denosumab Bioequivalents
Sandoz, renowned as a worldwide pioneer in the production of generic and biosimilar pharmaceuticals, disclosed that Wyost® (denosumab-bbdz) and Jubbonti®, both containing the active ingredient denosumab-bbdz, have received authorization from the US Food and Drug Administration. These are the inaugural biosimilar versions of denosumab granted approval by the FDA and they are sanctioned for all uses of the original reference drugs.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Keren Haruvi, the leader of Sandoz's operations in North America, remarked: "Sandoz has become the first to receive FDA endorsement for a biosimilar counterpart to denosumab, a pharmaceutical solution aimed at mitigating primary and secondary bone deterioration issues, including osteoporosis and cancer-induced bone complications that severely impact the well-being of individuals. I am honored that Sandoz is at the forefront, facilitating the availability of these transformative treatments for individuals in dire need."
Wyost® received the green light for the prevention of bone complications in individuals grappling with multiple myeloma and bone metastases stemming from solid malignancies. It is also sanctioned for use in both adults and bones of adolescents who have reached skeletal maturity to manage giant cell bone tumors that are not amenable to surgical interventions or where surgery could lead to significant detriment, as well as to tackle hypercalcemia of malignancy that is unresponsive to bisphosphonate therapy.
The skeletal structure ranks as the third most prevalent target for metastatic cancerous growths. While almost all cancer varieties have the potential to invade bone tissues, causing discomfort and bone fractures, the types most commonly associated with bone metastasis are breast and prostate cancers.
Jubbonti® has been authorized to administer treatment for postmenopausal women with osteoporosis who are at an elevated risk of fractures. It is also approved to augment bone density in men with osteoporosis who are similarly at a heightened risk of fractures, as well as in both genders who are enduring glucocorticoid-induced osteoporosis and possess a significant fracture risk. Further applications include escalating bone mass in men facing a high fracture risk while undergoing androgen deprivation therapy for non-metastatic prostate cancer and in women at a similar risk receiving adjuvant aromatase inhibitor therapy for breast cancer.
The recent nod of approval from the FDA for Jubbonti® is underpinned by extensive clinical trial data and comes with a set of cautionary directives on its labeling. Moreover, Jubbonti®'s authorization features the implementation of Sandoz's own Jubbonti® Risk Evaluation and Mitigation Strategy. This plan is structured to educate both healthcare providers and patients on the potential for severe hypocalcemia linked to using Jubbonti® in those with advancing chronic kidney ailments, which includes individuals who rely on dialysis.
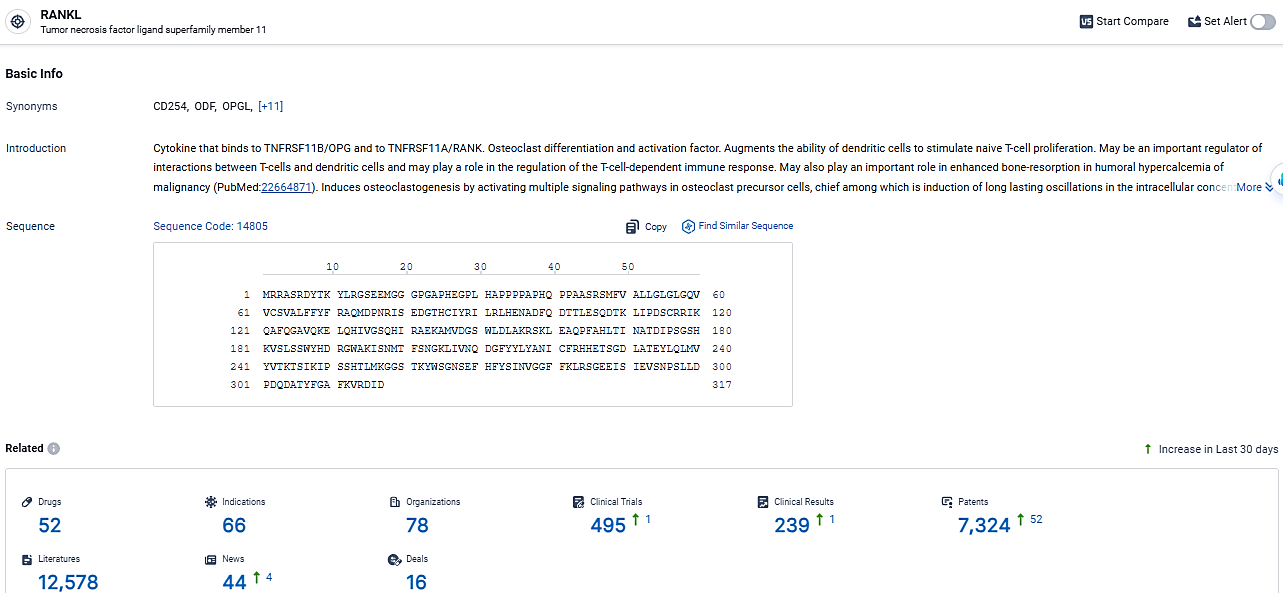
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 7, 2024, there are 52 investigational drugs for the RANKL target, including 66 indications, 78 R&D institutions involved, with related clinical trials reaching 495, and as many as 7324 patents.
Wyost® 120 mg/1.7 mL injection has been approved by the FDA as interchangeable with the reference medicine, a human monoclonal antibody designed to bind to the RANKL protein, an activator of osteoclasts. Wyost® is indicated in the US to prevent SREs in patients with multiple myeloma and in patients with bone metastases from solid tumors, to treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity, and to treat hypercalcemia of malignancy refractory to bisphosphonate therapy.