AbbVie Explores New Target ChemR23 for the Treatment of Chronic Inflammation
On February 28th, American pharmaceutical company AbbVie announced the establishment of a strategic partnership with France's OSE Immunotherapeutics to jointly develop a novel monoclonal antibody drug named OSE-230, which is aimed at treating chronic inflammation.
It is reported that OSE-230 is the world's first monoclonal antibody to target the activation of a member of the GPCR family, the chemerin receptor (ChemR23), and is anticipated to offer a new mechanism for addressing chronic inflammation issues and modulating the function of macrophages and neutrophils.
According to the terms of the collaboration agreement, AbbVie will obtain the exclusive global license and will be responsible for the further research and development, manufacturing, and commercialization of OSE-230. As part of the agreement, OSE Immunotherapeutics will receive an upfront payment of $48 million, and is also eligible to acquire up to an additional $665 million in subsequent payments upon the achievement of certain clinical development, regulatory approvals, and commercial sales milestones. Moreover, if the drug achieves net sales on a global scale, OSE Immunotherapeutics will be entitled to receive tiered royalties proportionate to these revenues.
About Chemerin
Chemerin was initially identified in 1997 and discovered to be present in psoriasis lesions, with increased expression following local application of the retinoid analog tazarotene. Consequently, it was initially named Tazarotene Induced Gene 2 (TIG2). Subsequent research indicated that retinoic acid receptors (RARs) could upregulate the expression of TIG2, thus the gene was termed Retinoic Acid Receptor Responder 2 (RARRES2). RARRES2 was once considered to be a soluble ligand capable of binding to cell surface receptors and participating in anti-proliferative effects.
By 2003, the protein sequence of RARRES2 was revealed, and it was officially named chemerin, while also confirming a G protein-coupled orphan receptor, ChemR23, as its functional receptor.
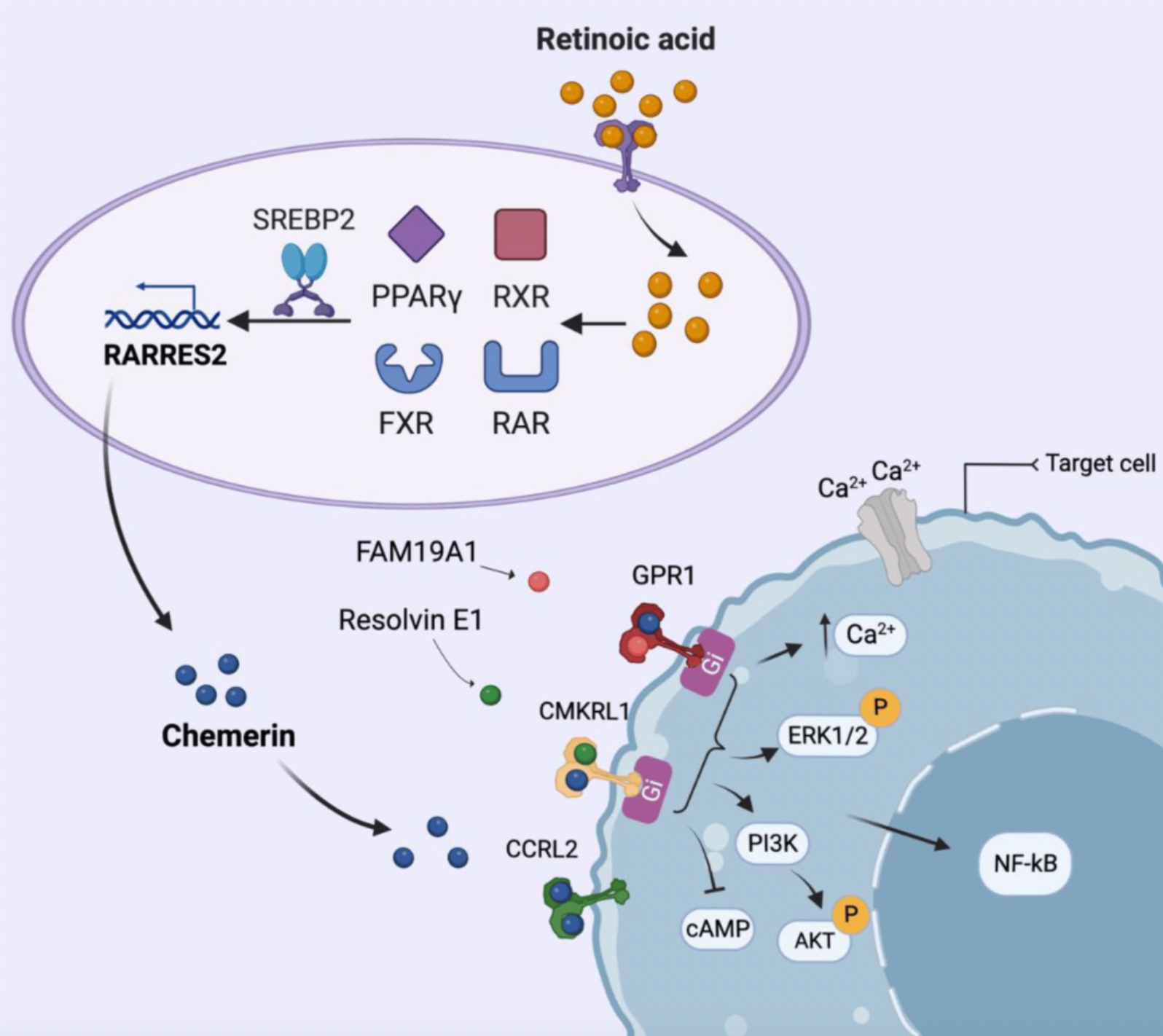
The study also discovered that two nuclear receptors capable of heterodimerizing with the Retinoid X Receptor (RXR), as well as a nuclear regulatory factor, can also influence the production of chemerin. For instance, the FXR agonist GW4064 is able to increase the expression of chemerin in HepG2 cells and primary hepatocytes, yet this effect is abolished after FXR knockout.
About ChemR23
ChemR23 (chemerin receptor 1) is the first identified member of the GPCR family from the chemerin lineage, which has been reported to be activated by two ligands: one is the peptide chemical, chemerin, and the other is the lipid mediator Resolvin E1 (RvE1), derived from eicosapentaenoic acid (EPA). Chemerin serves as a chemoattractant capable of attracting monocytes and macrophages to sites of inflammation, while RvE1 plays a role in promoting the resolution of inflammation by enhancing the phagocytosis of apoptotic neutrophils by macrophages, aiding in the resolution of inflammation.
ChemR23 was first reported in 1996. This receptor is primarily expressed in various tissues, including dendritic cells, monocytes, macrophages, endothelial cells, placenta, lungs, muscle, heart, adipose tissue, skin, and spleen. When chemerin binds to CMKLR1, it activates Gi protein, leading to decreased levels of cyclic adenosine monophosphate (cAMP), which in turn causes phosphorylation of extracellular-signal-regulated kinases 1/2 (ERK1/2) and activation of nuclear factor kappa B (NFκB), thus participating in the inflammatory response process.
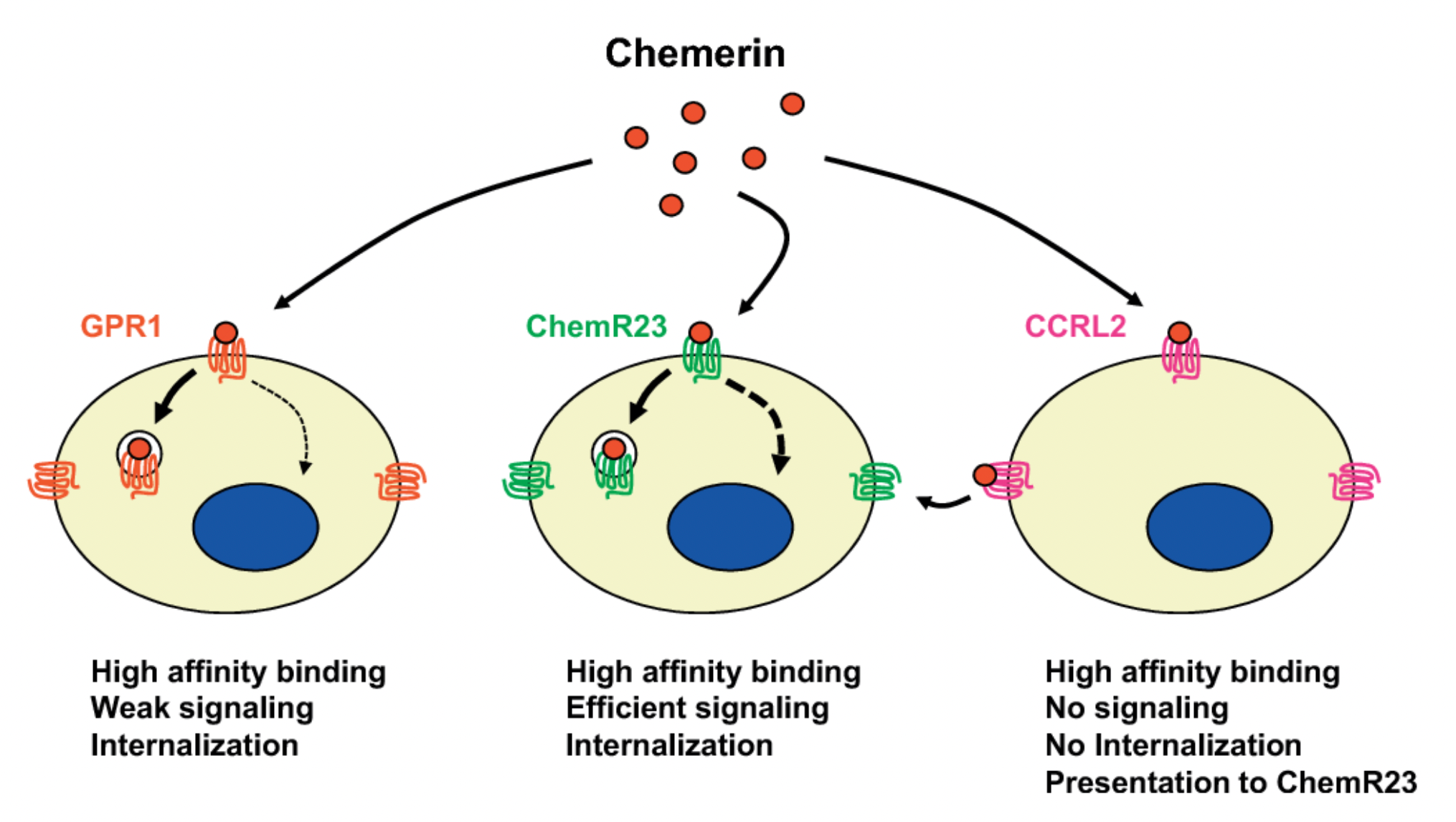
Aside from ChemR23, members of the Chemerin receptor family also include GPR1 (Chemerin receptor 2) and CCRL2.
Early research indicates that in un-polarized macrophages, the use of two promoters, P1 and P3, is relatively balanced. However, under the stimulation of lipopolysaccharide (LPS) or interferon-gamma (IFN-γ), the transcriptional activity of the P3 promoter increases in pro-inflammatory M1-type macrophages, leading to the upregulation of ChemR23 expression. These M1-type macrophages that express ChemR23 exhibit chemotactic responses to Chemerin, whereas M2-type macrophages, due to the lack of ChemR23 surface receptors, do not possess a responsive capability to Chemerin. The expression of ChemR23 is finely regulated by inflammatory and anti-inflammatory signals, playing a significant role in the conversion of human M1-type macrophages to inflammation-resolving macrophages mediated by Resolvin E1 (RvE1).
Chronic Inflammation and the Development of OSE-230
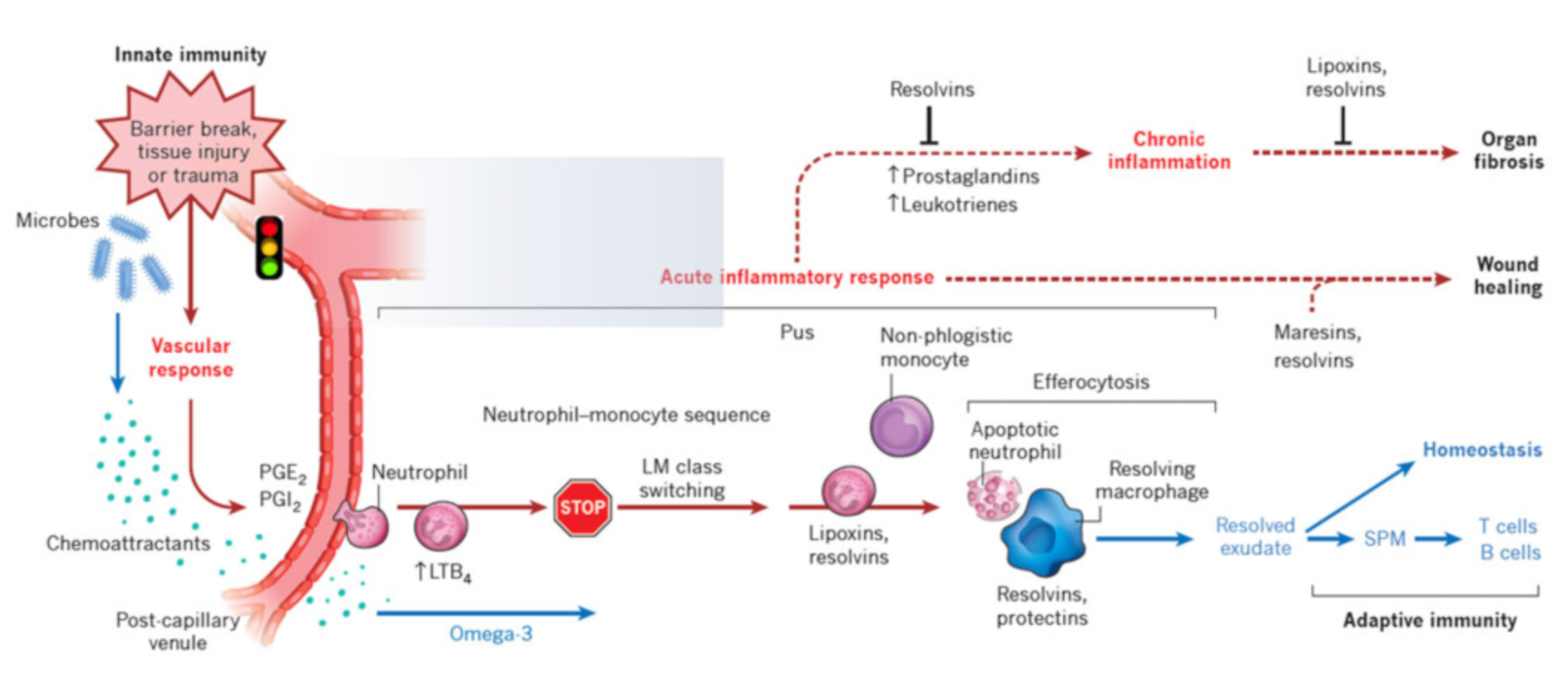
Persistent inflammation is a prominent feature of all chronic inflammatory or autoimmune diseases. If left uncontrolled or unresolved, ongoing inflammation can lead to further tissue damage and may trigger tissue fibrosis, ultimately resulting in the loss of organ function. The mechanism of action for most anti-inflammatory drugs is to suppress inflammation by blocking pro-inflammatory pathways.
OSE Immunotherapeutics is a comprehensive biotechnology company dedicated to developing and co-developing immunotherapies for the control of immunological cancers and immune-inflammatory diseases.
OSE Immunotherapeutics is developing a pioneering new drug called OSE-230. This medication is expected to facilitate the effective resolution of inflammation by driving the affected tissues to complete their own inflammatory processes, thereby restoring tissue integrity. In contrast to traditional therapies that only suppress the inflammatory response, OSE-230 is designed to guide the natural progression of inflammation towards resolution and repair, thus more effectively treating and reversing the pathological tissue changes caused by chronic inflammation.
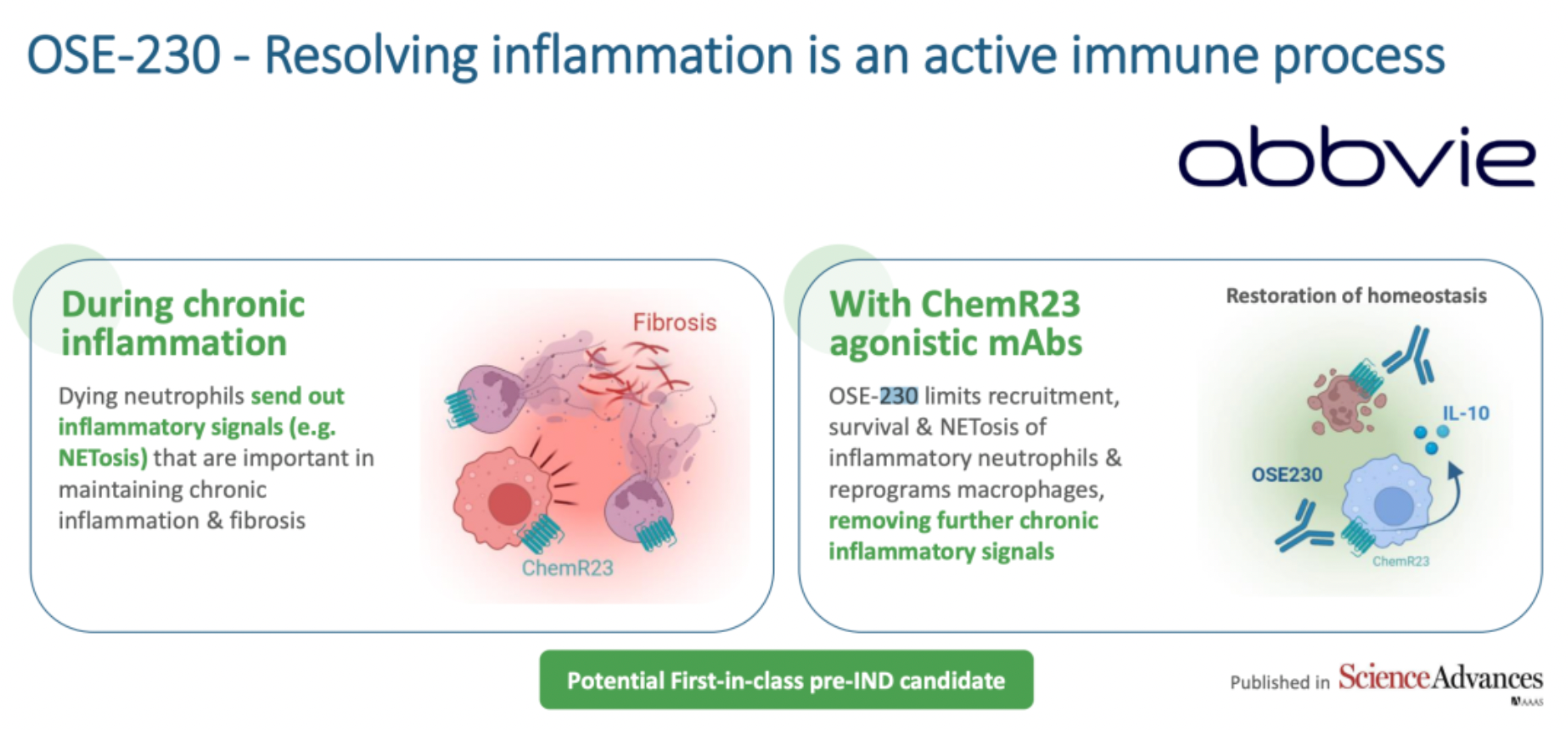
In 2019, OSE Immunotherapeutics submitted for the first time a patent on anti-ChemR23 monoclonal antibodies and their clinical applications, presenting research results on several anti-ChemR23 monoclonal antibodies in animal models of autoimmune diseases, spontaneous colitis, Type 1 diabetes, sepsis, and cancer.
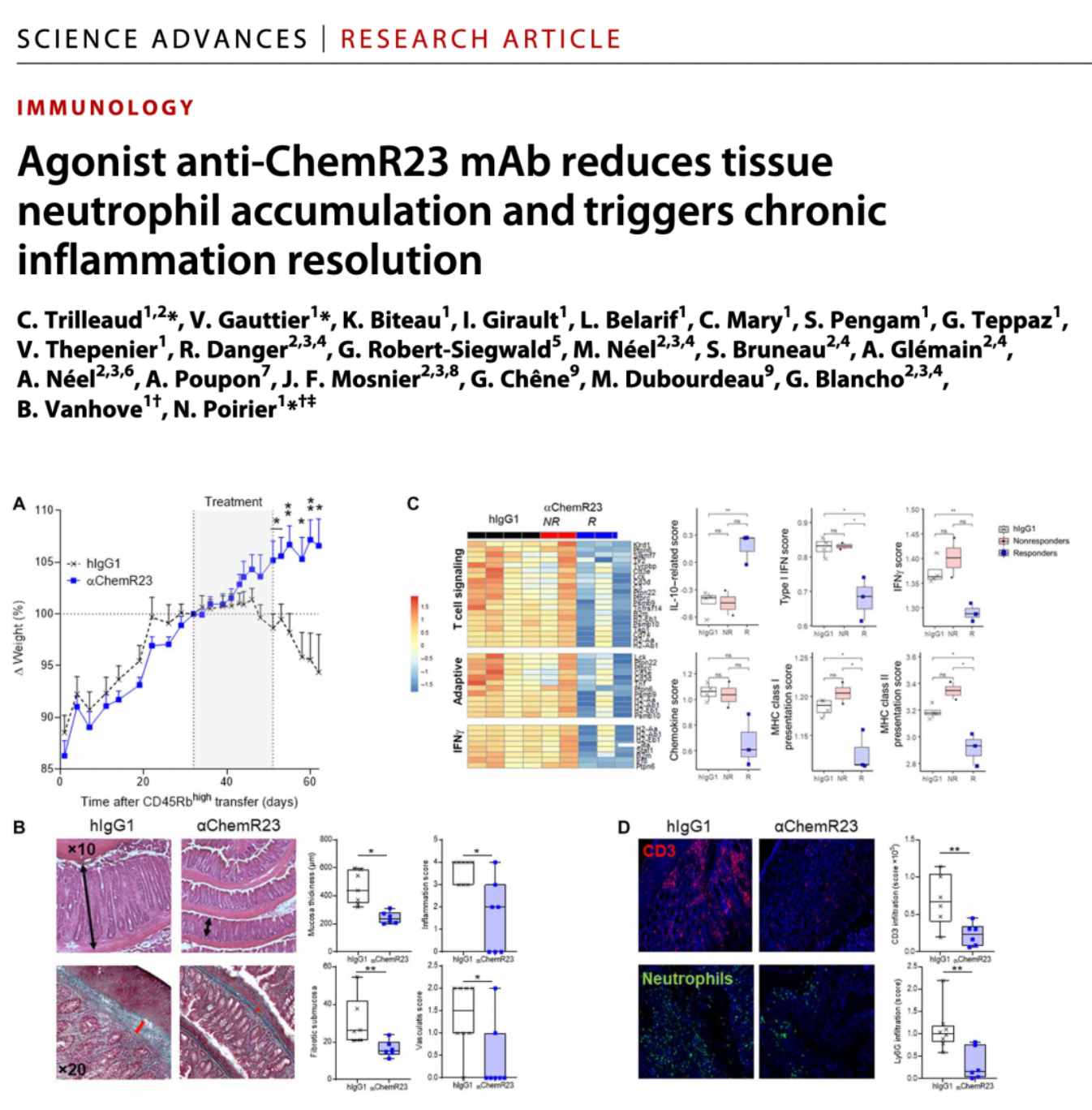
In 2021, the team at OSE Immunotherapeutics first published partial preclinical research findings on OSE-230 in the journal Science Advances. This monoclonal antibody (mAb) targeting ChemR23 accelerated the resolution of acute inflammation in models and initiated the resolution process in ongoing chronic colitis models, significantly reducing tissue damage, fibrosis, and the rate of inflammation-driven tumorigenesis. These studies suggest that the suboptimal effects of current IBD therapies may be associated with neutrophil infiltration, and ChemR23 represents a promising therapeutic target for treating chronic inflammation.
In 2023, the OSE Immunotherapeutics team once again published an article, investigating the therapeutic potential of targeting the resolving inflammation receptor ChemR23. The study results indicated that the expression level of ChemR23 was higher in macrophages induced by M-CSF and tumor cell supernatants, resembling tumor-associated macrophages, compared to macrophages differentiated by GM-CSF. Activation of ChemR23 with antibodies was able to profoundly modulate macrophages of the M-CSF type and those resembling tumor-associated macrophages, affecting their surface marker characteristics, cytokine secretion, gene mRNA expression, and immunological functions.
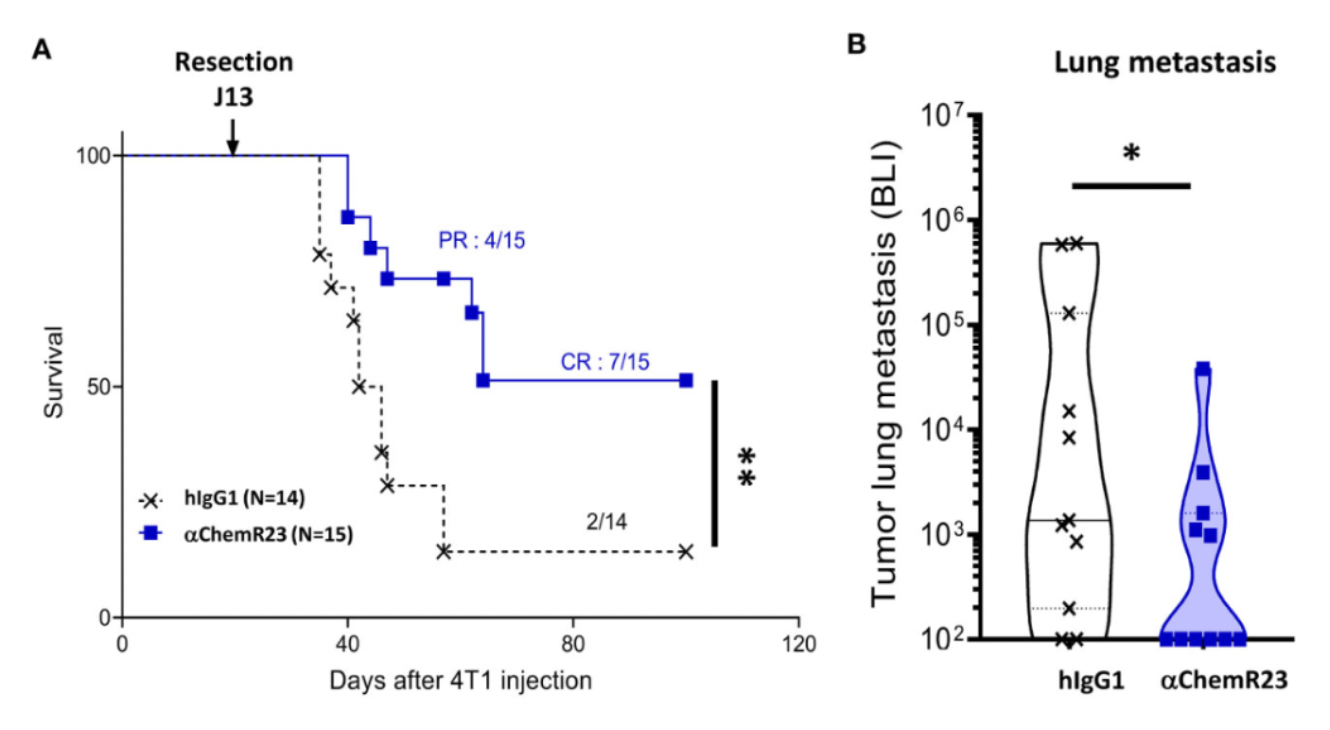
These findings unveil an attractive strategy, namely, by targeting ChemR23 and its mediated resolving inflammation pathway, it might be possible to overcome the pro-tumorigenic effects of tumor-associated macrophages, thus offering a novel approach to cancer therapy.