Sanofi ends tusamitamab ravtansine Phase 3 trial for 2nd-line NSCLC due to unmet primary endpoint
Sanofi is halting its worldwide clinical trial endeavors for the drug tusamitamab ravtansine. This cessation comes in light of results from a planned interim assessment conducted during the Phase 3 CARMEN-LC03 study. The study aimed to assess the efficacy of tusamitamab ravtansine as a single-agent therapy against docetaxel in patients with advanced metastatic non-squamous non-small cell lung cancer. Specifically, the trial focused on individuals displaying elevated concentrations of the carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) within their tumors.
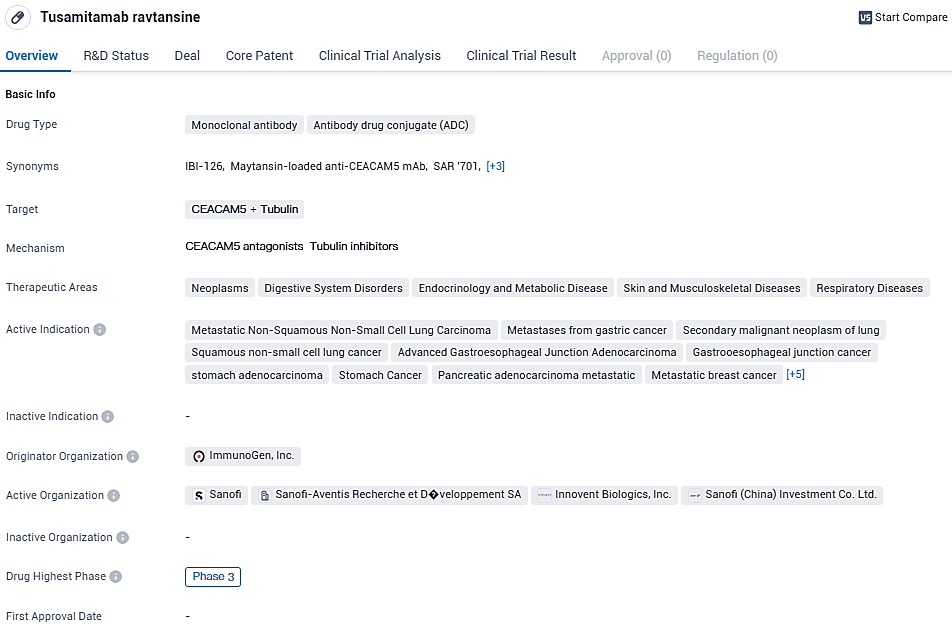
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
An external Data and Safety Monitoring Board concluded that the use of tusamitamab ravtansine in solitary treatment failed to achieve its dual main goal of lengthening the time without cancer progression when compared with the chemotherapy agent docetaxel. In spite of a perceived trend toward longer survival times, the decision to discontinue the project was made after it was determined that there was no significant improvement in progression-free survival upon the concluding evaluation.
The safety outcomes for tusamitamab ravtansine were consistent with what was documented before, showing a reduced frequency of significant categories of side effects when compared to docetaxel. Participants in the study will be given the option to continue with their existing treatment if it proves beneficial, as judged by their healthcare provider, or switch to a generally accepted therapy for their condition.
Dietmar Berge, the leading medical executive and development director, expressed, "Our dedicated team extends its sincerest thankfulness to everyone engaged in the tusamitamab ravtansine research efforts. Despite the setbacks, our determination to push forward with innovative treatments in cancer areas with critical needs remains strong. We are committed to further investigating CEACAM5 as a potential cancer marker, especially in cancers where its presence is significantly pronounced."
Sanofi is set to pursue further investigation into the potential of tusamitamab-based ADCs and continue CEACAM5 studies in various cancerous conditions. CEACAM5 belongs to a larger group of 12 glycoprotein family members, playing potential roles in cellular bonding and movement, acting as an inhibitor to cell death processes, and commonly found in elevated levels across numerous forms of cancer.
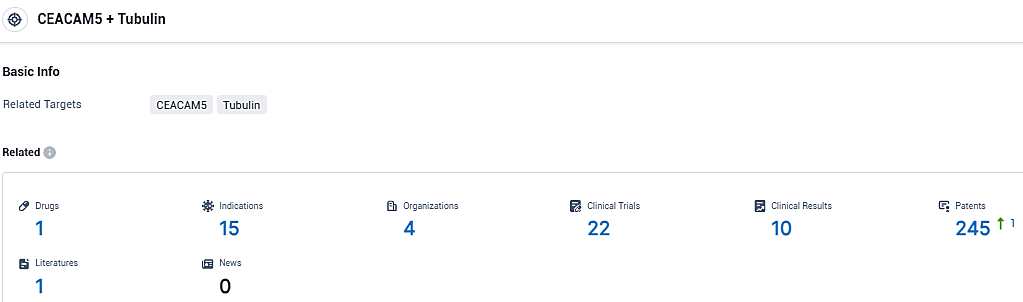
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 29, 2023, there are 1 investigational drugs for the CEACAM5 and Tubulin target, including 15 indications, 4 R&D institutions involved, with related clinical trials reaching 22, and as many as 245 patents.
As an ADC, Tusamitamab ravtansine combines the specificity of a monoclonal antibody with the cytotoxic effects of a conjugated drug. This approach allows for targeted delivery of the drug to cancer cells, potentially minimizing off-target effects and improving efficacy.