Santhera's Vamorolone secures EU approval for Duchenne Muscular Dystrophy therapy
Santhera Pharmaceuticals has declared that its drug, AGAMREE® (vamorolone), has received authorization within the European Union to be prescribed for those diagnosed with Duchenne muscular dystrophy. This approval is applicable to patients who are at least four years old, without restrictions on the specific genetic mutation or their ability to walk.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The European Medicines Agency has recognized the substantial safety advancements of AGAMREE in terms of preserving normal bone metabolism, density, and growth when contrasted with traditional corticosteroid treatments. Furthermore, patients transitioning from conventional corticosteroids to AGAMREE showed sustained therapeutic effects while experiencing recovery in both growth and bone health.
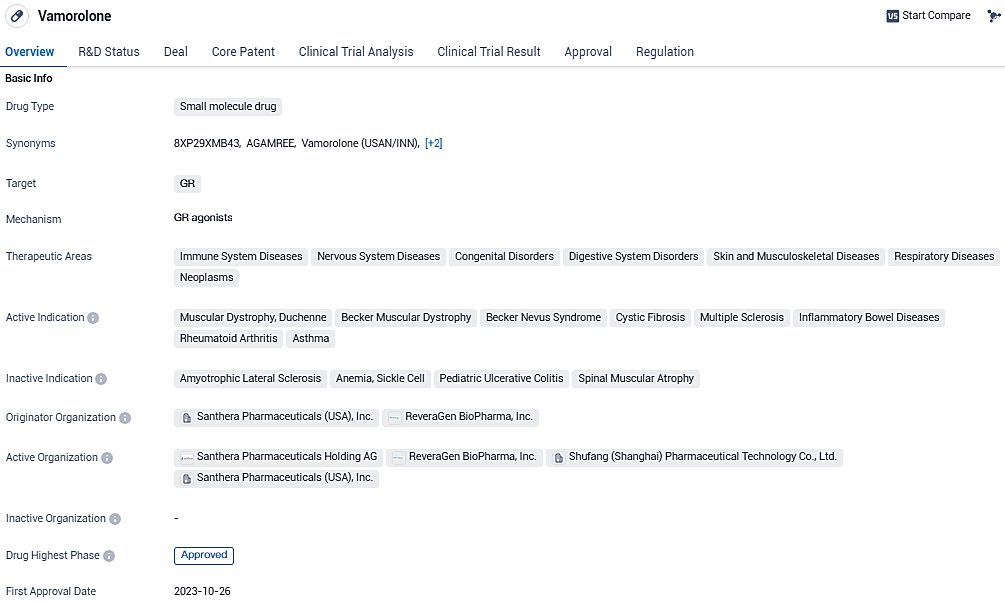
AGAMREE has now become the premier pharmaceutical to be granted comprehensive endorsement by the EU and, following clearance from the U.S. Food and Drug Administration in October, it is also the debut therapy sanctioned in both the U.S. and the EU for DMD patients.
"We are overjoyed with the EC's approval to distribute AGAMREE within the EU market. This underscores the drug's superior safety profile and its capacity to improve bone health and growth compared to standard corticosteroids," expressed Dario Eklund, Santhera's Chief Executive Officer. "Our team is now dedicated to swiftly introducing AGAMREE to Duchenne patients, targeting an initial market introduction in Germany at the beginning of 2024."
"The World Duchenne Organization is pleased to witness the progression of AGAMREE. The active participation of numerous Duchenne patient groups was crucial as they offered initial funding and contributed to the development of clinical trials, including their design, desired outcomes, and participant enrollment. This has culminated in the first comprehensive market approval for a DMD treatment in the European Union," stated Elizabeth Vroom, Chair of the World Duchenne Organization.
The endorsement from the EC came after the Committee for Medicinal Products for Human Use delivered a favorable assessment on October 13, 2023. The marketing authorization will be operative across the 27 EU member countries as well as in Iceland, Liechtenstein, and Norway. Santhera is preparing for the product's initial launch in Germany in early 2024.
On October 27, 2023, Santhera disclosed that the FDA had given its approval for AGAMREE oral suspension at a concentration of 40 mg/ml for the United States market for use in treating individuals with DMD who are 2 years old and above. Catalyst Pharmaceuticals obtained exclusive rights to AGAMREE in the North American region and is aiming for a release in the United States in the first quarter of 2024.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
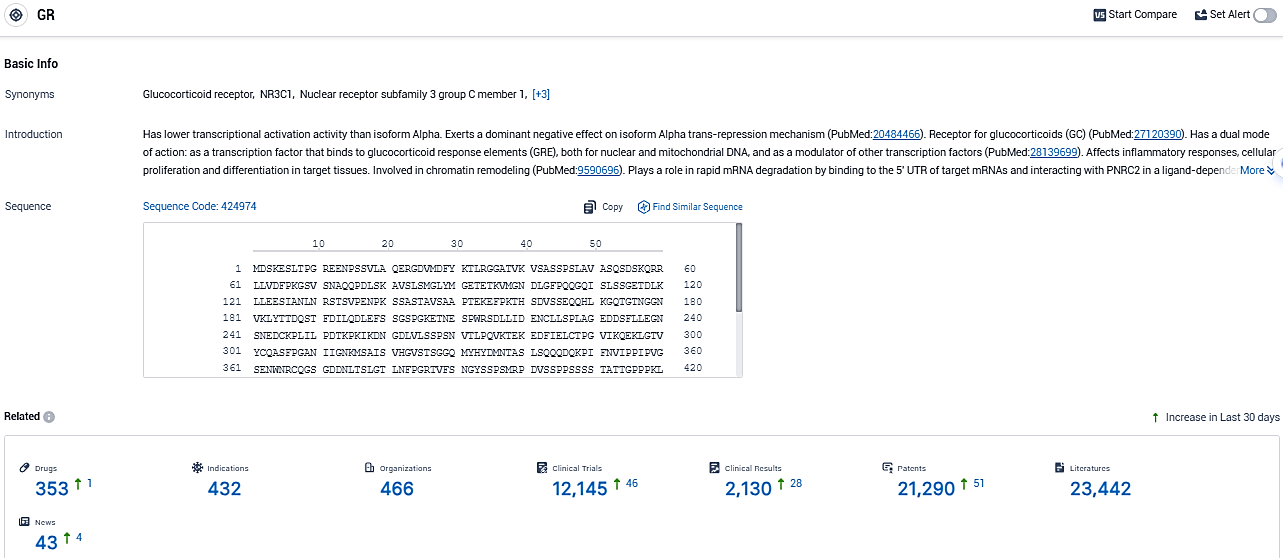
According to the data provided by the Synapse Database, As of December 27, 2023, there are 353 investigational drugs for the GR target, including 432 indications, 466 R&D institutions involved, with related clinical trials reaching 12145, and as many as 23442 patents.
Vamorolone is a small molecule drug that targets the glucocorticoid receptor and is being developed for the treatment of various diseases. AGAMREE® has Orphan Drug status for DMD in the U.S. and in Europe and has received Fast Track and Rare Pediatric Disease designations by the U.S. FDA and Promising Innovative Medicine status from the UK MHA for DMD. Vamorolone is approved for use in the United States and the European Union.