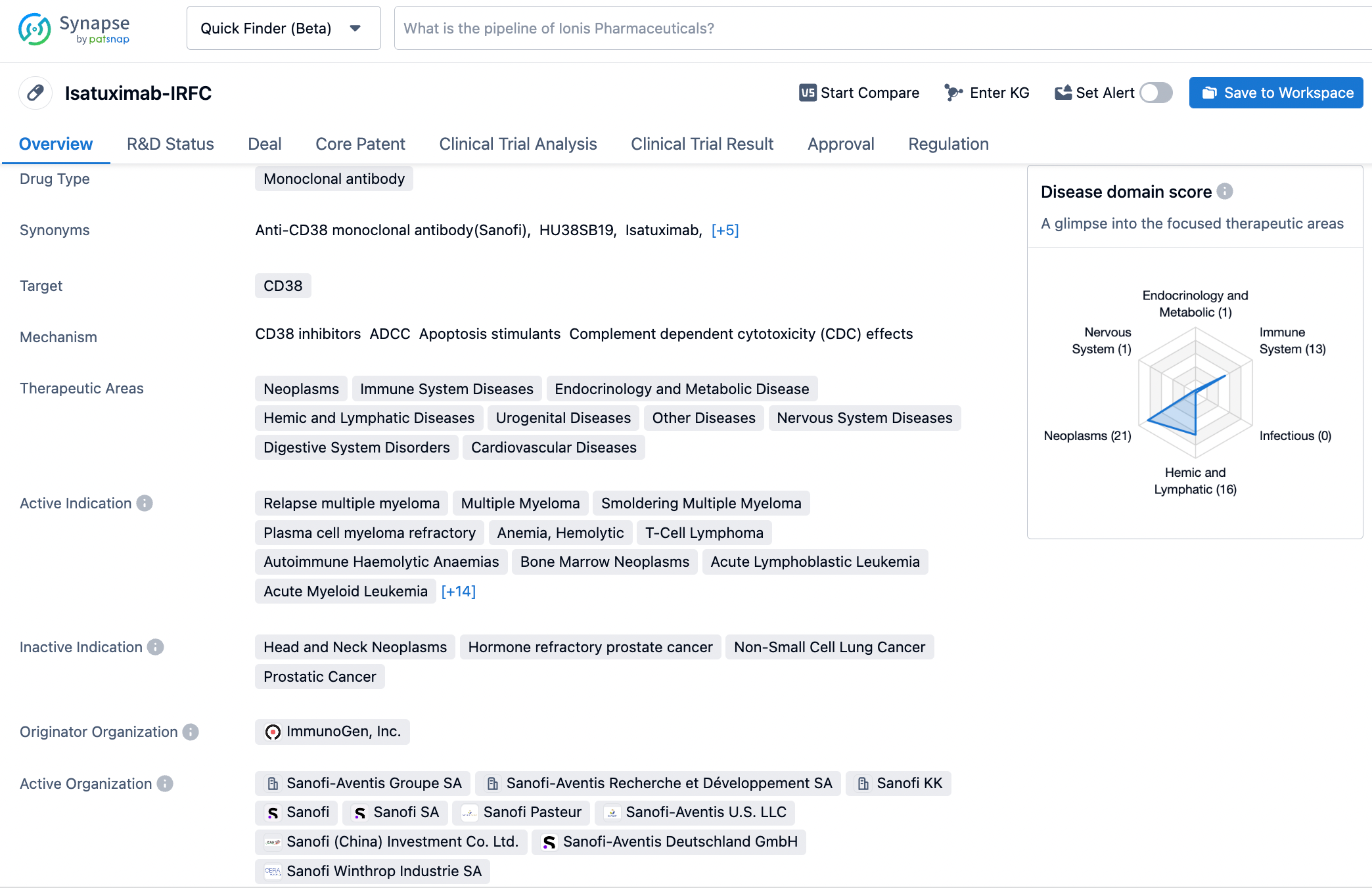
Sarclisa® (isatuximab) Phase 3 trial slows progression in new multiple myeloma patients ineligible for stem cell transplant
The IMROZ Phase III clinical study, which is assessing the experimental combination of Sarclisa (isatuximab) with the conventional treatment regimen of bortezomib, lenalidomide, and dexamethasone, achieved its main objective during a scheduled interim efficacy analysis. The results indicated a notable enhancement in delaying disease progression, with statistical significance, in comparison to treatment with VRd only in non-transplant candidates with newly identified multiple myeloma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The results from the IMROZ study appear to be encouraging for individuals managing early-stage multiple myeloma who cannot undergo a transplant. This patient group urgently requires new viable treatment alternatives. Choosing the right initial treatment is pivotal for patients, particularly for those not eligible for transplants, due to the decline seen with further treatment regimens. Thierry Facon, MD, an associate of the French Academy of Medicine and the principal investigator for IMROZ, remarked.
Dietmar Berger, MD, PhD, the Chief of Global Development at Sanofi, expressed, “Following a second Phase 3 analysis, we've observed Sarclisa outperform the established standard treatment in patients facing their first encounter with the disease. This reinforces our conviction in Sarclisa as a standout therapy. The findings reflect our dedication to pushing the boundaries of research for those affected by multiple myeloma, and we are eager to provide a more elaborate depiction of how Sarclisa can enhance the patient journey when used in earlier treatment stages.”
Conducted across 104 sites in 21 countries, the phase 3, multi-centered, and open-label IMROZ trial recruited 484 patients grappling with early-stage multiple myeloma who were ineligible for a transplant. During the trial, Sarclisa was distributed intravenously at a 10 mg/kg ratio. For the inaugural 42-day cycle, the doses were weekly, and from the second to the fourth cycle, every two weeks. It was used in unison with the subcutaneous application of bortezomib, orally taken lenalidomide, and dexamethasone administered either intravenously or orally.
The principal objective of the IMROZ trial focuses on the duration of progression-free survival. Its critical secondary aims involve the rate of complete response, the proportion of minimal residual disease negativity in subjects with complete responses, the incidence of a very good partial response or superior, and the length of overall survival.
Additional outcomes assessed are the total response rate, time until disease progression, duration of the response achieved, lapse until the initial response, interval until the optimal response, progression-free survival upon receiving subsequent treatments, progression-free survival as determined by MRD status, the rate of sustained MRD negativity reaching or surpassing 12 months, tolerability, pharmacokinetic attributes, immunogenic reactions, specific disease and quality of life measurements, symptoms linked to the disease and its treatment, utility of health states, and overall health conditions.
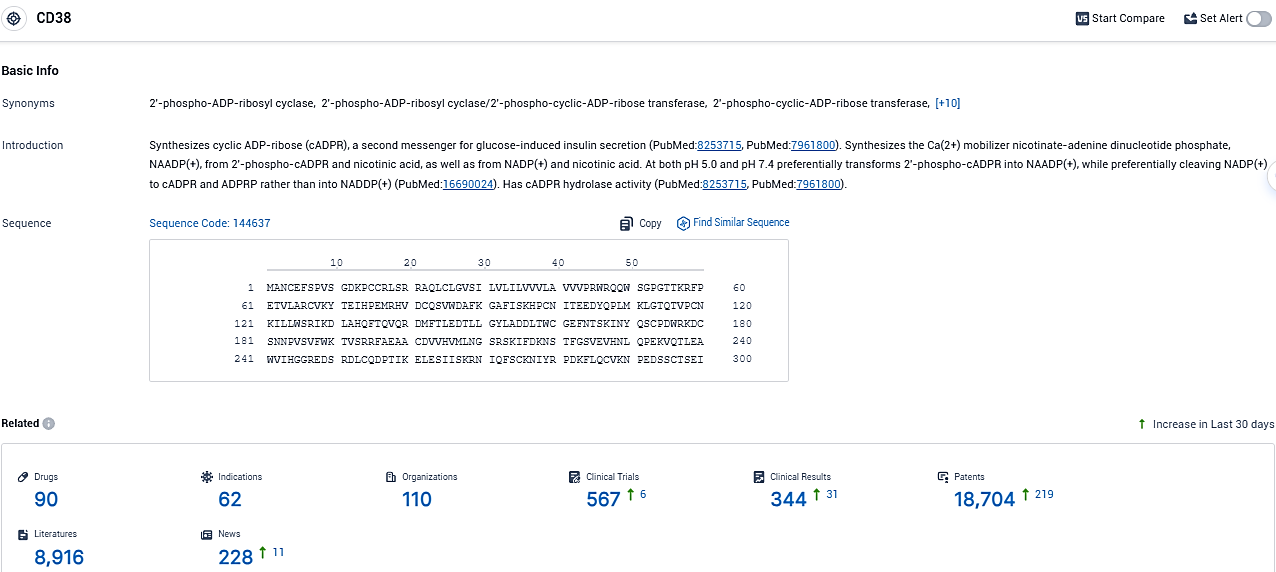
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 13, 2023, there are 90 investigational drugs for the CD38 target, including 62 indications, 110 R&D institutions involved, with related clinical trials reaching 567, and as many as 18704 patents.
Sarclisa is a monoclonal antibody that binds to a specific epitope on the CD38 receptor on multiple myeloma cells, inducing distinct antitumor activity. In the U.S., the generic name for Sarclisa is isatuximab-irfc, with irfc as the suffix designated in accordance with Nonproprietary Naming of Biological Products Guidance for Industry issued by the U.S. Food and Drug Administration.