Sarepta Therapeutics Releases Preliminary Findings of EMBARK, a Critical Global Trial on ELEVIDYS, a Gene Treatment for Duchenne Muscular Dystrophy
Sarepta Therapeutics, Inc., a pioneer in targeted genetic therapies for uncommon conditions, has revealed preliminary findings from EMBARK, a worldwide Phase 3 clinical trial. This double-blind, placebo-controlled study was designed to evaluate ELEVIDYS (delandistrogene moxeparvovec-rokl) in patients aged 4 to 7 suffering from Duchenne muscular dystrophy.
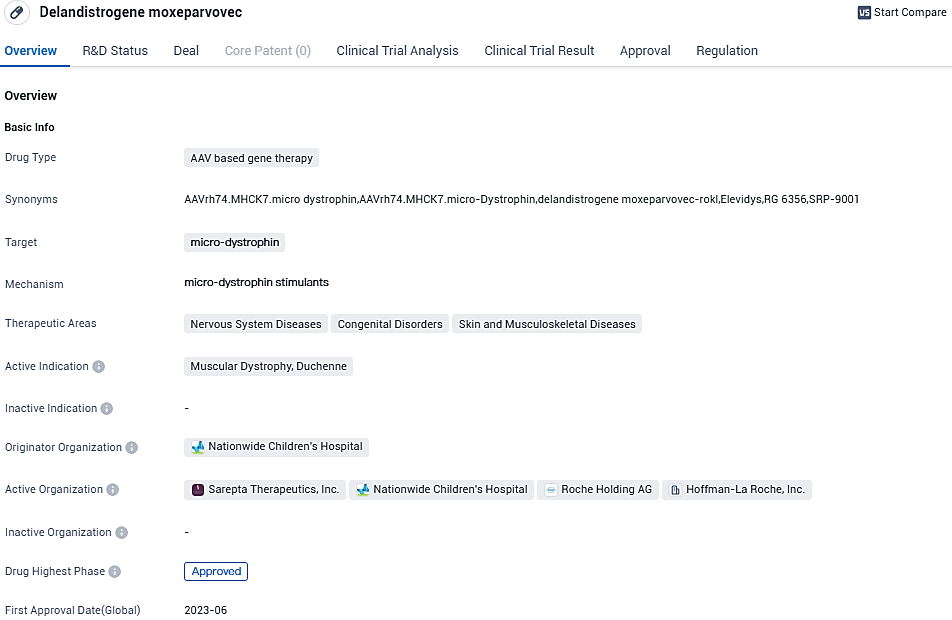
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"The EMBARK study, a double-blind, placebo-controlled trial we conducted, substantiates the assertion that ELEVIDYS alters Duchenne's course and offers advantages to patients of all age groups who are grappling with this devastating progressive disease. ELEVIDYS surpassed all expectations in all endpoints of the research, reaching statistical significance in all predetermined key secondary endpoints, and throughout all age subgroups within the key secondary endpoints.” stated Doug Ingram, Sarepta's CEO and president.
Doug Ingram further commented, “Our next step, based on the EMBARK findings, is to promptly submit a request for an update to widen the treatment access to all patients. Crucially, we've communicated the EMBARK top-tier results to FDA leadership, who have indicated their willingness to consider label expansion based on a comprehensive data review, and expressed their intention to speedily evaluate our submission.”
“The power of time to rise as a strong prognostic tool and the significant implications of crossing the 5 second marker in forecasting functional deterioration and prospective loss of mobility, are undisputed in natural history.1 The EMBARK study revealed a significant clinical relevance of ELEVIDYS treatment in curbing the progression beyond this critical juncture,” commented Craig McDonald, M.D., Chair and Professor at UC Davis Health Department of Physical Medicine and Rehabilitation, and a contributing investigator in the EMBARK study.
“Treatment efficacy is evidenced by the consistency of the positive impact across all timed function checks and age brackets. Additionally, it is noteworthy that this was the only clinical trial to date to document a significant and meaningful enhancement on the 95th percentile stride speed, sourced from an objective community wearable activity monitor, a pioneering metric used in DMD trials" McDonald added.
The EMBARK study didn't flag any novel safety concerns, consolidating ELEVIDYS' manageable safety portfolio. The primary treatment-related side effects were pyrexia and gastrointestinal issues. Seven participants encountered a treatment-related SAE and no significant alterations were noticed in SAEs tied to known ELEVIDYS' risks.
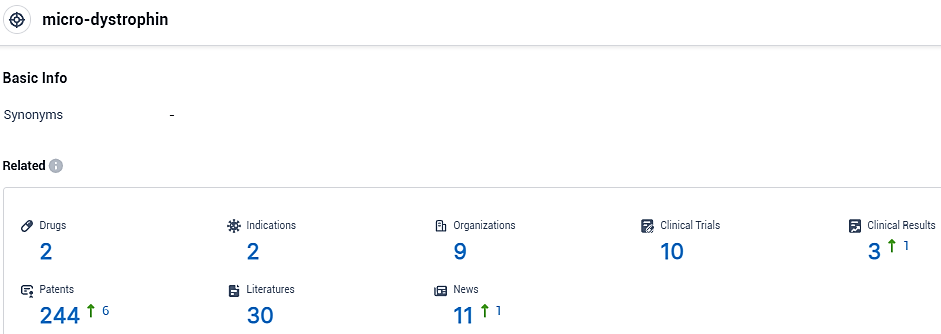
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 3, 2023, there are 2 investigational drugs for the micro-dystrophin target, including 2 indications, 9 R&D institutions involved, with related clinical trials reaching 10, and as many as 244 patents.
ELEVIDYS acts as a one-time gene transfer therapy, intended for intravenous administration, engineered to tackle the root cause of Duchenne muscular dystrophy by promoting the targeted synthesis of ELEVIDYS micro-dystrophin in skeletal muscle. It is intended for the management of ambulatory pediatric patients suffering from Duchenne muscular dystrophy and has received expedited approval based on the observed micro-dystrophin expression in the skeletal muscle of patients who were administered ELEVIDYS.