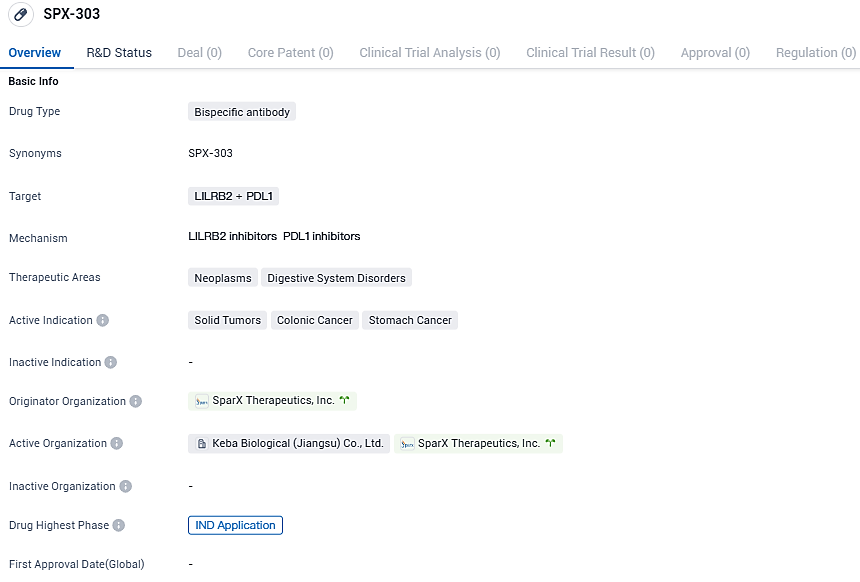
SparX Biopharma declares FDA approval of IND application for SPX-303, an initial human test of anti-LILRB2/PD-L1 antibody medication
In a significant progression in the field of powered antibody therapies, SparX Biopharmaceutical Corp. joyfully declares the FDA's endorsement of its IND application for SPX-303, a pioneering anti-LILRB2/PD-L1 bispecific antibody drug contender. This recognition propels SparX's relentless dedication to provide innovative solutions to patients contending with progressed or refractory solid tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dr. Gui-Dong Zhu, the CEO of SparX Biopharmaceutical, expressed his elation: "Winning the FDA's approval for our IND application represents a pivotal milestone in our continuous drive towards revolutionizing cancer therapy paradigms. The pre-research findings for SPX-303 not only hint at potential, but bolster our conviction to exploit its medicinal potential in a clinical milieu, predominantly for those patients who had earlier had finite therapy alternatives."
Thanks to the FDA's support, SparX Biopharmaceutical is perfectly positioned to initiate a Phase 1 clinical trial, intending to evaluate the safety, acceptability, and initial effectiveness of SPX-303 in patients distressed by advanced or stubborn solid tumors. This human test aims to see the medicinal potential of SPX-303, possibly signalling the start of a new era in inventive and enhanced medical results.
Formulated by SparX, SPX-303 is a pioneering bispecific antibody treatment, artfully constructed to involve two key immune checkpoint proteins, LILRB2 and PD-L1, at once. Playing an important role in maintaining the immune system's balance and vitality, these checkpoints help prevent autoimmune disorders.
Whilst LILRB2 mainly regulates overactive inborn immune responses, keeping the body's inbuilt defense system in balance, PD-L1—often excessively found in tumor cells—carefully manages T cell activation, ensuring an accurate immune response. Yet, cancer often tricks these immune checkpoints, establishing a protective mechanism against immune strike.
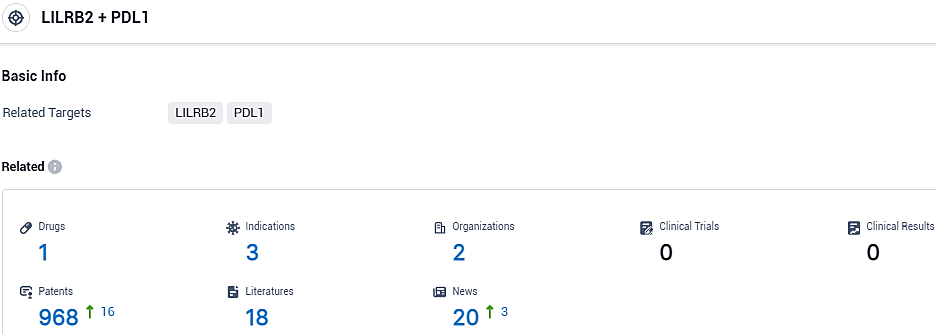
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 3, 2023, there are 1 investigational drugs for the LILRB2 and PD-L1 target, including 3 indications, 2 R&D institutions involved, and as many as 968 patents.
SPX-303 is painstakingly engineered to break down this barrier, enabling the immune system to detect and passionately destroy cancerous cells, thus creating a revolutionary path in cancer treatment.