Satellos Submits Regulatory Filing to Begin Phase 1 Trial for SAT-3247
Satellos Bioscience Inc., a publicly traded biotech firm focused on creating innovative small molecule therapies to enhance muscle disease and disorder treatments, revealed on July 10, 2024, that it has submitted a clinical research proposal to an Australian Human Research Ethics Committee. This submission seeks regulatory approval through the Therapeutic Goods Administration (TGA)’s Clinical Trial Notification scheme to initiate a first-in-human Phase 1 clinical trial for SAT-3247.
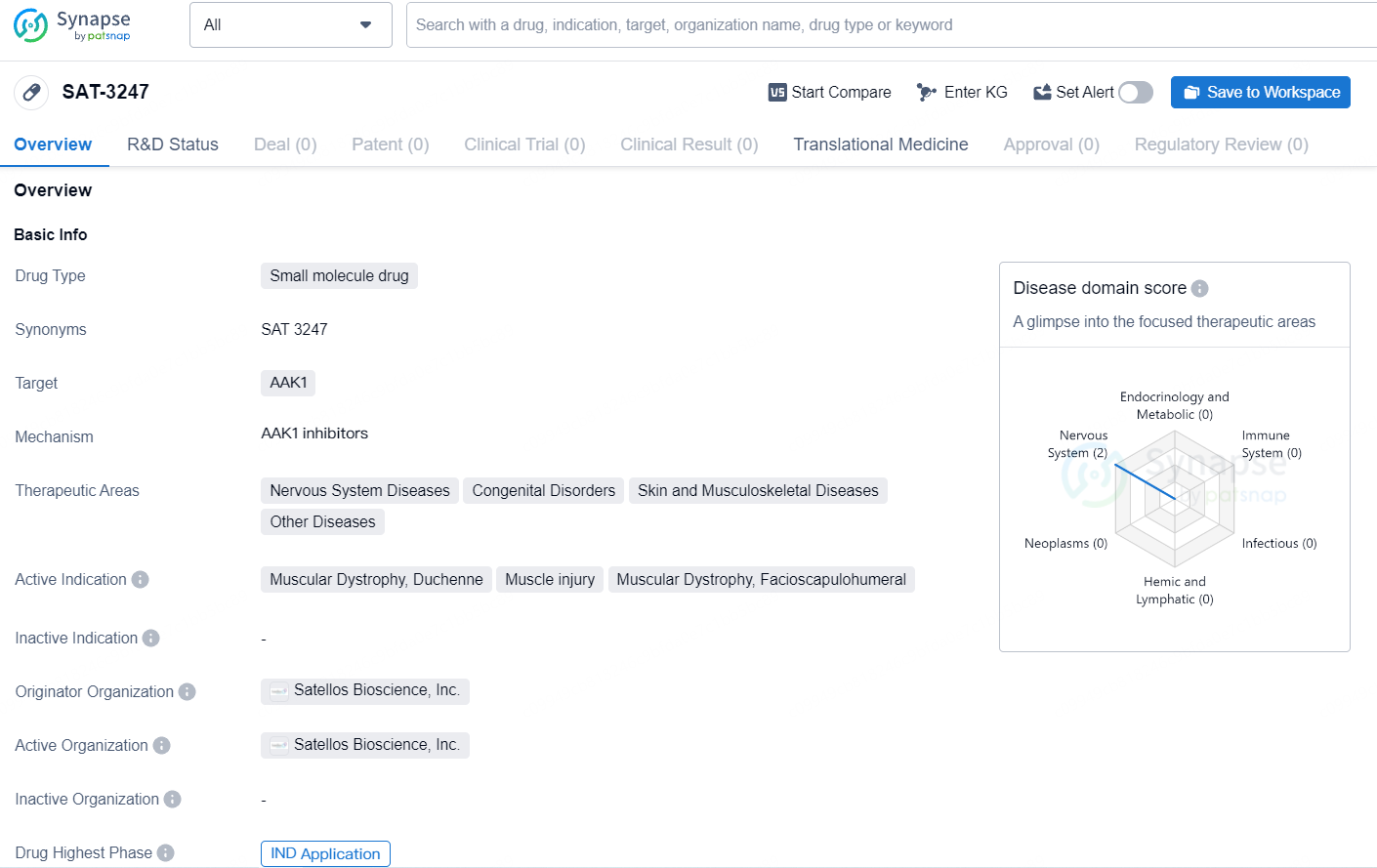
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“We consider this a pivotal advancement for Satellos as we transition into a clinical-stage enterprise,” stated Frank Gleeson, CEO and Co-founder of Satellos. “Our team is delighted to arrive at this critical juncture, a goal we have been diligently working towards over the past year. We believe this accomplishment will serve as a springboard for a series of future milestones.”
SAT-3247 is Satellos' proprietary, orally administered small molecule drug, developed to promote the regeneration of skeletal muscle lost due to Duchenne muscular dystrophy and other degenerative conditions or injuries. Subject to HREC approval and CTN acceptance by Australia's TGA, the Phase 1 clinical trial will enlist healthy participants to evaluate the safety and pharmacokinetic characteristics of SAT-3247. Upon successful completion of this phase, Satellos intends to progress SAT-3247 into clinical trials involving DMD patients starting in early 2025.
“We are encouraged by our preclinical study outcomes and eager to commence clinical development of SAT-3247,” remarked Phil Lambert, Ph.D., Chief Scientific Officer of Satellos. “Before submitting our regulatory documents, we performed preclinical and toxicological evaluations according to standards set by relevant global regulatory authorities. Consequently, we anticipate utilizing these results in support of further Phase 1 trials and subsequent clinical studies in Australia and other regions, including the United States and Canada, where we aim to initiate trials with DMD patients.”
SAT-3247 is a small molecule drug candidate for oral administration, designed to address the fundamental cause of muscle deterioration in degenerative diseases, primarily in Duchenne. SAT-3247 offers a novel mechanism of action aimed at restoring impaired muscle regeneration due to the lack of functional dystrophin.
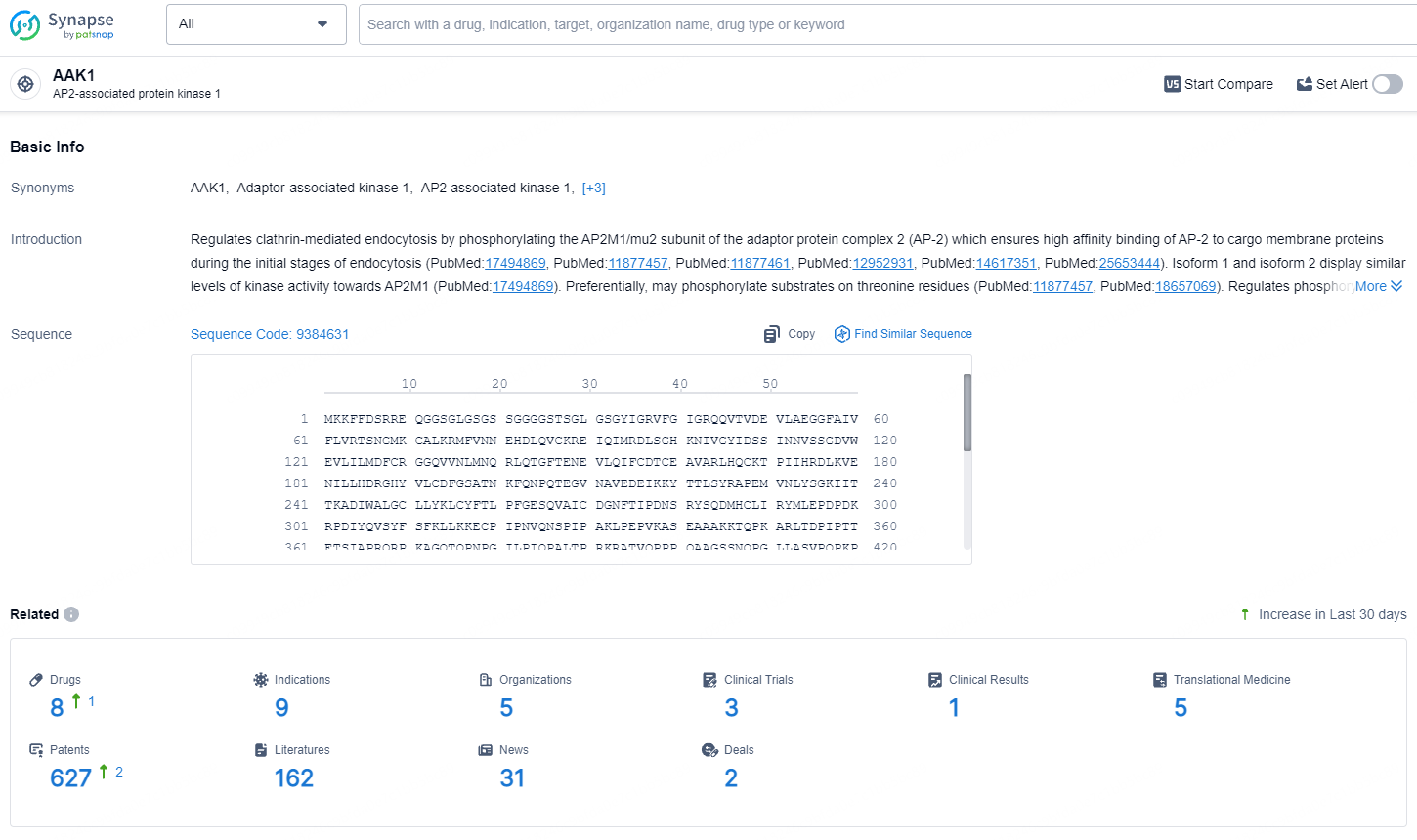
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 16, 2024, there are 8 investigational drugs for the AAK1 target, including 9 indications, 5 R&D institutions involved, with related clinical trials reaching 3, and as many as 627 patents.
SAT-3247 shows promise in potentially providing therapeutic benefits for patients suffering from a range of muscular and neurological disorders. As a small molecule drug targeting AAK1, it has the potential to address unmet medical needs in these areas. Further clinical trials will be necessary to assess the drug's safety and efficacy in treating these conditions.