Schrödinger Presents SGR-3515 Preclinical Results at 2024 ENA Symposium
Schrödinger (Nasdaq: SDGR) shared new preclinical findings on SGR-3515, its experimental Wee1/Myt1 inhibitor, during a poster session at the 36th EORTC-NCI-AACR Symposium (ENA 2024). The findings indicate that in preclinical models, the administration of SGR-3515 leads to enhanced anti-tumor effects, resulting in more significant and longer-lasting responses compared to treatments focused solely on Wee1 or Myt1 inhibitors. Additionally, the preclinical results suggest that SGR-3515 possesses a beneficial pharmacological profile and dosing regimen, making it suitable for investigating intermittent dosing strategies in patients.
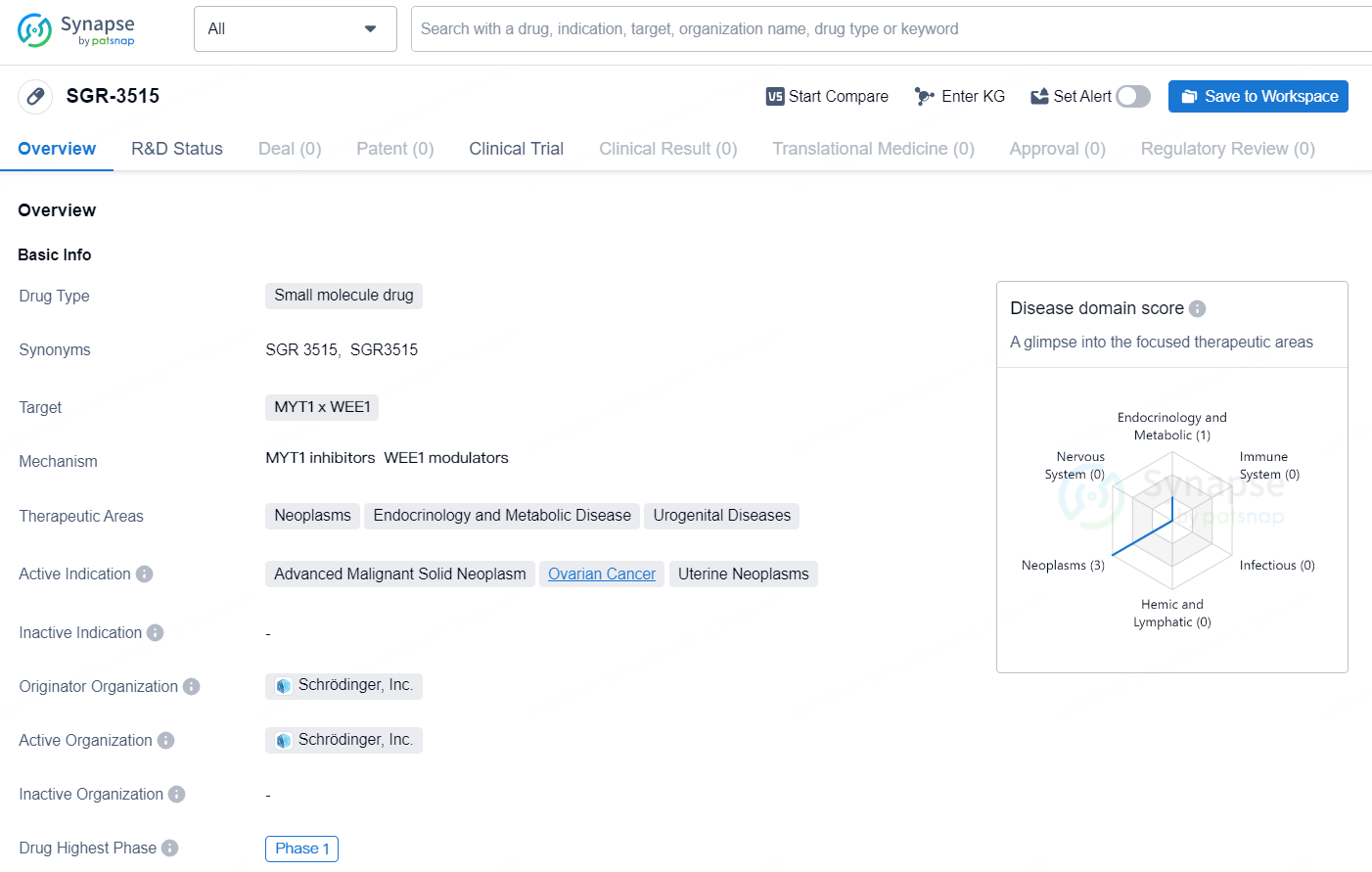
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Wee1 and Myt1 kinases play a critical role in the regulation of the cell cycle and the DNA damage response, providing a necessary delay for DNA repair prior to cell division. The simultaneous inactivation or suppression of both Wee1 and Myt1 leads to a specific sensitivity in cancer cells, a phenomenon known as synthetic lethality, which is emerging as a potential therapeutic approach for various cancers. A Phase 1 dose-escalation trial for SGR-3515 is currently underway for patients with advanced solid tumors in the United States and Canada, with initial findings from this clinical trial anticipated in late 2025.
Additionally, Schrödinger plans to showcase preclinical findings from its PRMT5-MTA initiative during a poster presentation on October 25. Researchers at Schrödinger have discovered a new group of selective and potent PRMT5-MTA inhibitors and are currently refining lead compounds intended for utilization in peripheral and brain tumors.
“We are excited to present these promising preclinical findings on SGR-3515, which may offer a best-in-class solution for patients facing a variety of solid tumors, including uterine and ovarian cancers, where there is a significant unmet need,” said Karen Akinsanya, Ph.D., president of R&D therapeutics at Schrödinger. “We also eagerly anticipate sharing preclinical data regarding the discovery of a new series of compounds within our PRMT5-MTA inhibitor initiative. These projects demonstrate our commitment to utilizing our computational platform extensively to develop highly refined molecules aimed at treating diseases with considerable medical needs, and we are optimistic about the future of our therapeutics portfolio.”
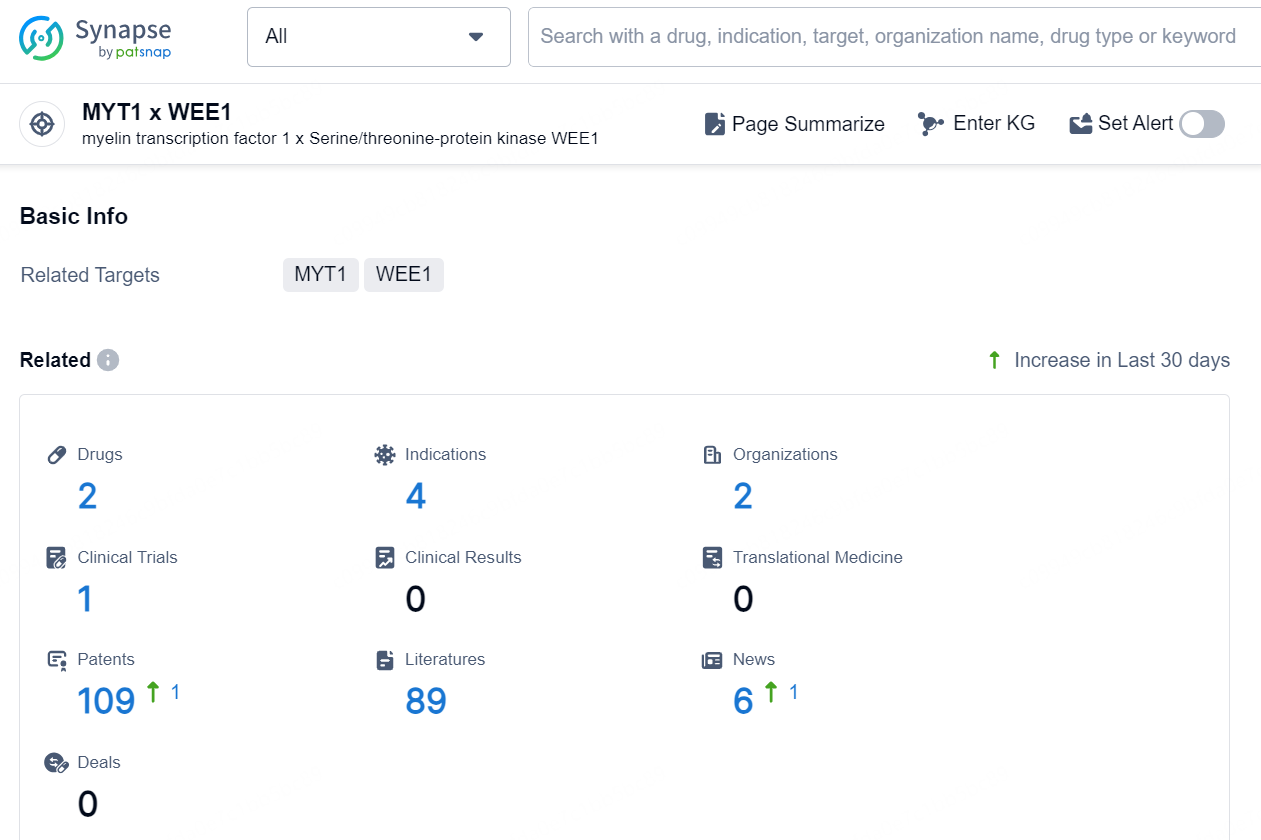
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 2 investigational drugs for the MYT1 and WEE1 targets, including 4 indications, 2 R&D institutions involved, with related clinical trial reaching 1, and as many as 109 patents.
SGR-3515 is a small molecule drug developed by Schrödinger, Inc. It targets MYT1 and WEE1 and is being developed for the treatment of neoplasms, endocrinology and metabolic diseases, as well as urogenital diseases. The drug is actively indicated for the treatment of advanced malignant solid neoplasm, ovarian cancer, and uterine neoplasms.