Semi-Annual Leqvio® Boosts Statin Efficacy in Lowering Cholesterol in Real-World Care
Novartis has revealed fresh findings indicating that integrating Leqvio (inclisiran) administration biannually into an established regimen of statins, taken to the highest bearable extent and before incorporating ezetimibe as suggested in clinical guidelines, leads to a considerable decrease in low-density lipoprotein (LDL) cholesterol levels. This effect was observed in a practical clinical environment among individuals suffering from atherosclerotic cardiovascular diseases (ASCVD). The data particularly highlighted its efficacy for patients with prior ASCVD incidents who were unable to achieve their targeted LDL cholesterol levels with only statins.
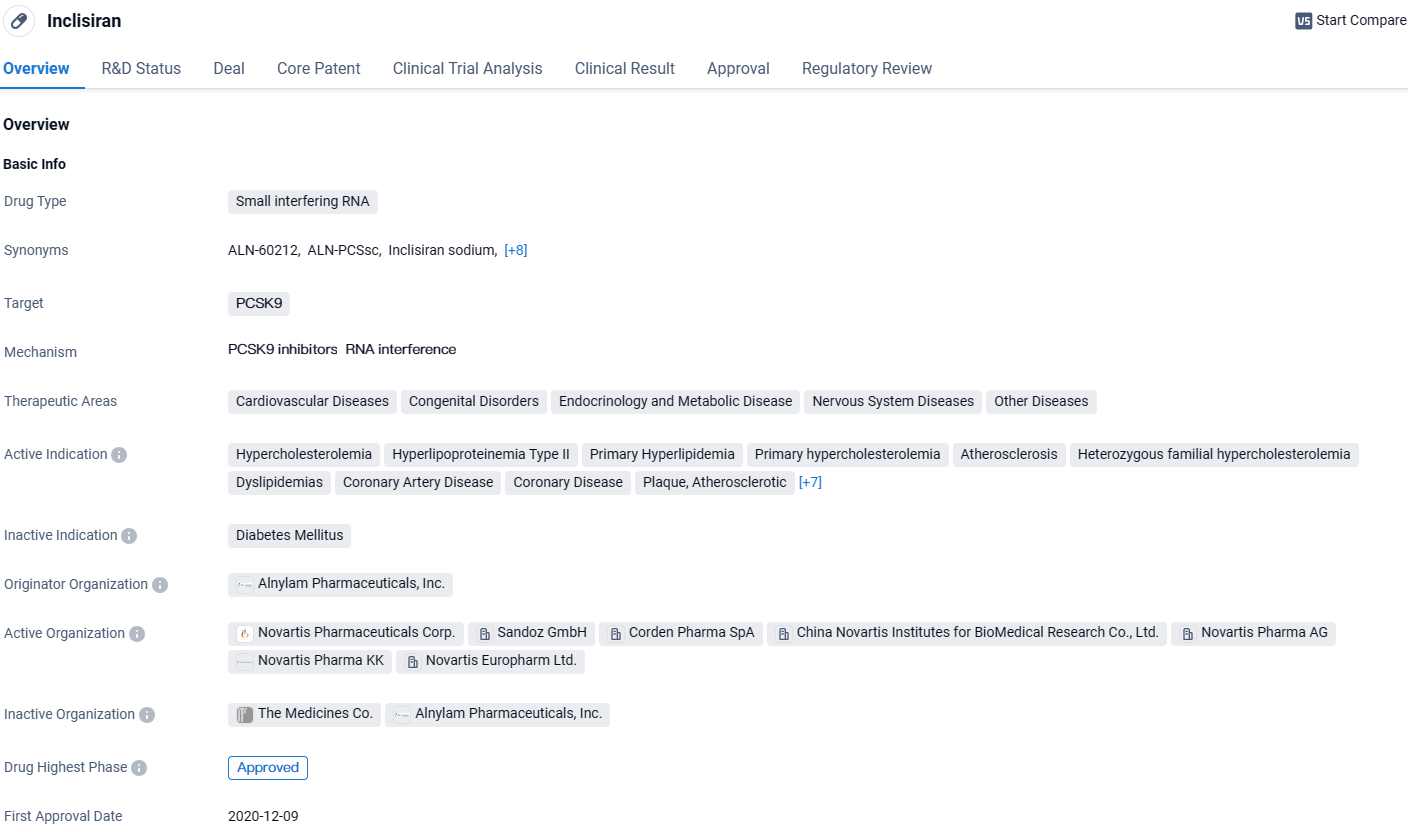
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The recently disclosed findings were showcased at the prestigious 2024 gathering of the American College of Cardiology's Annual Scientific Session & Expo, with simultaneous publication in the esteemed Journal of the American College of Cardiology.
"Through V-INITIATE, we examined a strategy for addressing the prevalent issue in clinical care—numerous ASCVD patients not attaining their recommended LDL-C targets with statins alone and the significant underuse of potent non-statin options," stated Dr. Michael Koren, the study's lead researcher, who serves as Medical Director and CEO at Jacksonville Center for Clinical Research.
“With an imperative to intensify LDL-C management, V-INITIATE's findings indicate that introducing proven non-statin alternatives such as Leqvio earlier in the treatment process can dramatically lower LDL-C levels for ASCVD patients struggling to achieve or maintain targeted levels,” Koren further remarked.
The safety outcomes from the V-INITIATE research were in harmony with those of the pivotal Phase III clinical trials and the ongoing open-label extension studies, ORION-3 and ORION-8, confirming the enduring safety profile of up to six years of treatment.
Dr. David Soergel, Novartis' Global Head of Cardiovascular, Renal and Metabolic Drug Development, noted, “V-INITIATE's data stand as a testament to the benefits of commencing novel non-statin treatments like Leqvio early on for ASCVD patients, which could enhance how we manage LDL-C reduction. This research complements the VictORION program's extensive evidence base by supplying real-world insights about Leqvio, a biannual HCP-administered treatment, and underscores its substantial clinical value.”
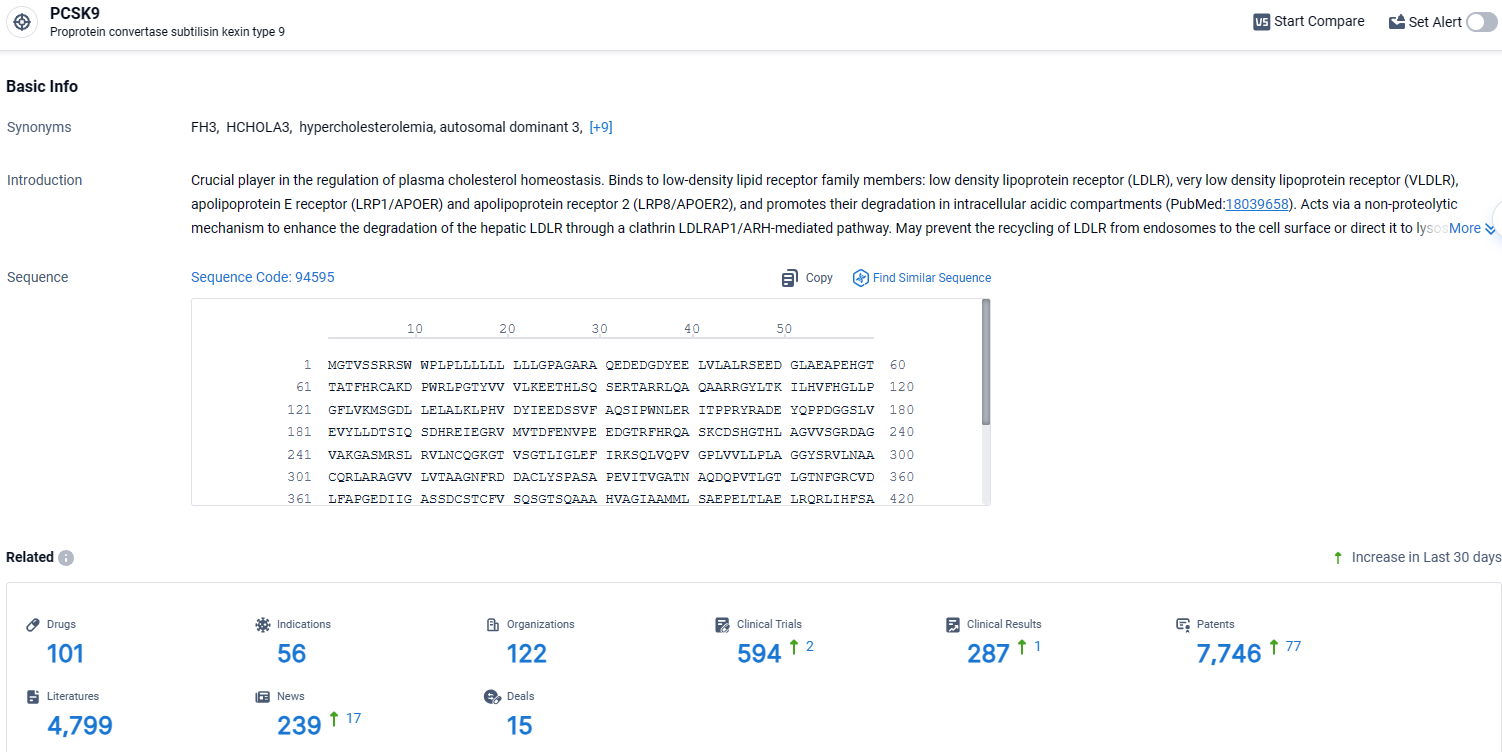
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 8, 2024, there are 101 investigational drugs for the PCSK9 target, including 56 indications, 122 R&D institutions involved, with related clinical trials reaching 594, and as many as 7746 patents.
Inclisiran is a small interfering RNA drug that targets PCSK9, a protein involved in cholesterol metabolism. As Inclisiran continues to gain regulatory approvals and enters the market, it has the potential to make a significant impact on the management of hypercholesterolemia and associated conditions. Its orphan drug status highlights its potential to address unmet medical needs in specific patient populations. Further research and real-world evidence will be crucial in assessing the long-term efficacy and safety profile of Inclisiran, as well as its potential benefits in various therapeutic areas beyond cardiovascular diseases.