Senti Bio granted FDA approval for SENTI-202, targeting resistant blood cancers like Acute Myeloid Leukemia
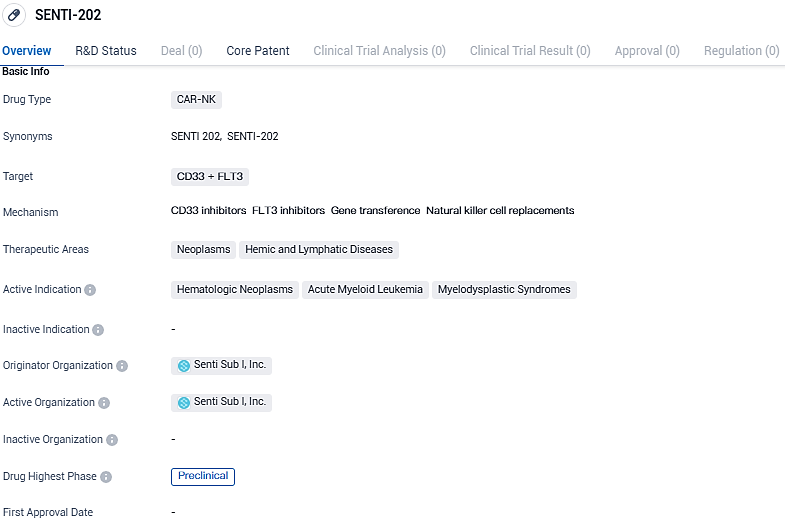
Senti Biosciences, Inc. has disclosed that the U.S. Food and Drug Administration (FDA) has approved its request to move forward with the new drug investigation for SENTI-202. This particular therapy is an engineered off-the-shelf product involving chimeric antigen receptor natural killer (CAR-NK) cells. The therapeutic agent is specifically crafted to identify and eradicate cancerous cells in the blood that display CD33 and/or FLT3 markers, all the while ensuring that the cells which constitute healthy bone marrow remain unharmed.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The firm is set to launch an early-stage clinical assessment for SENTI-202, planning to conduct it across various locations in the United States and Australia beginning in 2024. The inaugural subject is anticipated to receive treatment in Q2 of 2024. This exploratory study will focus on determining the optimal dosage, testing either one billion or 1.5 billion SENTI-202 cells injected post lymphodepleting pre-treatment in grown individuals who have recurrent or resistant blood cancers expressing CD33 and/or FLT3, acute myeloid leukemia included.
The initial administration involves a trio of weekly injections succeeding lymphodepletion. Patients may also have the opportunity for additional cycles of lymphodepletion and SENTI-202 cell infusions, with decisions guided by preliminary safety and effectiveness findings.
"With the approval of our Investigational New Drug application for SENTI-202, we've reached a pivotal point, signaling a key accomplishment for Senti as we evolve into a clinical-phase entity," announced Senti Bio's CEO and co-founder, Timothy Lu, MD, PhD.
"Our personnel has been tirelessly innovating our Gene Circuit technology, transforming what began as a conceptual approach in synthetic biology into an actual product poised to impact oncology. The advent of Senti’s inaugural clinical evaluation is eagerly anticipated, as is building upon this progress in the forthcoming period," Lu further commented.
By the close of 2024, the firm anticipates releasing preliminary results on the effectiveness from the phase 1 study, and insights into the long-term outcomes are expected in 2025. Thanks to a prearranged partnership with GeneFab, the firm has settled the bulk of production costs associated with this trial.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
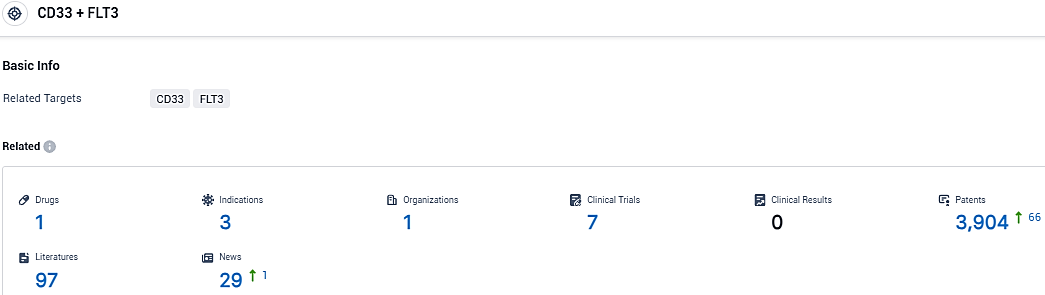
According to the data provided by the Synapse Database, As of December 30, 2023, there are 1 investigational drugs for the CD33 and FLT3 target, including 3 indications, 1 R&D institutions involved, with related clinical trials reaching 7, and as many as 3904 patents.
SENTI-202 targets CD33 and FLT3 and is intended for the treatment of hematologic neoplasms, acute myeloid leukemia, and myelodysplastic syndromes. Currently, the drug is in the preclinical phase, and further research and development are required before it can progress to human clinical trials.