Tyra Biosciences gave the first dose of TYRA-200 and reported promising progress with their TYRA-300 development
Tyra Biosciences, Inc., an enterprise operating at the clinical phase within the biotech industry, has made a public announcement regarding the commencement of its Phase 1 investigational study named SURF201 for their compound TYRA-200. This study is a critical step in advancing their pipeline of precision therapeutic agents designed to harness the potential of FGF Receptor biology. Moreover, the company has shared encouraging progress concerning its orally administered compound specifically targeting FGFR3, branded as TYRA-300.
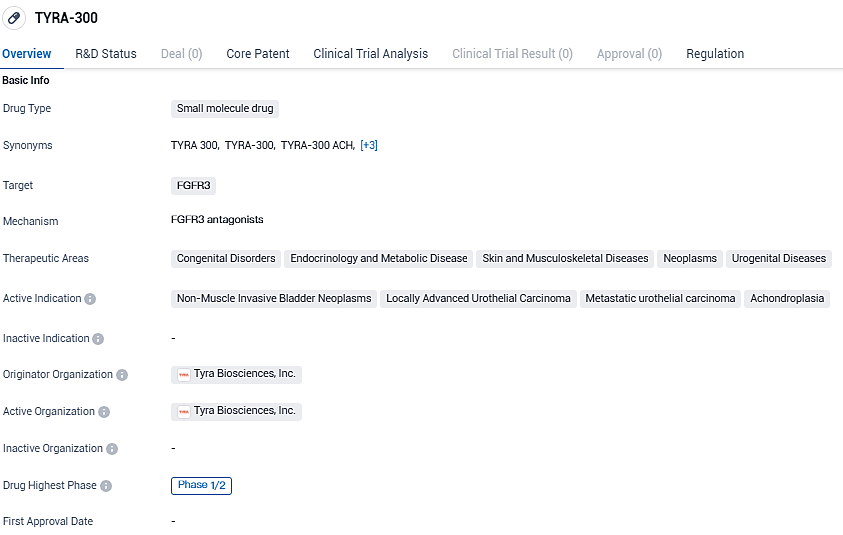
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Drawing near the conclusion of 2023, my conviction stands that TYRA occupies the strongest footing we've ever achieved. Currently, our portfolio boasts several programs in the clinical phase, particularly with TYRA-300 and TYRA-200, and the insights gleaned from SURF301 lend credence to our commitment to pioneering an industry-leading compound in TYRA-300. This will potentially enhance treatment success for children afflicted with achondroplasia as well as oncological patients," stated TYRA's Chief Executive Officer Todd Harris.
Harris further stated, "As we enter 2024, our enthusiasm is bolstered by our latest advancements, and we aim to press forward with our project roster, aiming towards key landmarks we are confident will produce substantive benefits for both our patient community and investors."
The initial stage of the SURF301 study is set to yield data that will guide the configuration of various dosing patterns and intervals for TYRA-300 for forthcoming research on expansive metastatic bladder cancer, localized bladder cancer not involving muscle tissue, and achondroplasia. Thus far, the first part of Phase 1 within the SURF301 trial maintains its progress in dose elevation, having successfully scaled several dosage groups that exceed the predicted dosages for the following Phase 2 study targeting pediatric achondroplasia.
TYRA is in the process of orchestrating a Phase 2 controlled trial with varied dosage groups using TYRA-300 for juvenile patients with achondroplasia. The pivotal goal of this research is to gauge both safety and tolerability among this demographic and to pinpoint the dosages for subsequent trial phases. Additional aims will contemplate the evaluation of alterations in growth pace, the proportionality of growth, the dynamics of drug absorption and distribution in the body, together with gauging life quality and examining biomarkers to deduce the dosage-efficacy relationship for TYRA-300.
TYRA anticipates that this forthcoming investigation will initially scrutinize treatment-naïve children between 5 and 12 years of age to ascertain the most effective dosage window and will also factor in an independent examination of similarly aged children experiencing achondroplasia who have not shown improvement following prior growth acceleration interventions. TYRA is preparing to file a proposal for an Investigational New Drug with the US FDA in the latter half of 2024, marking the commencement of the Phase 2 trial.
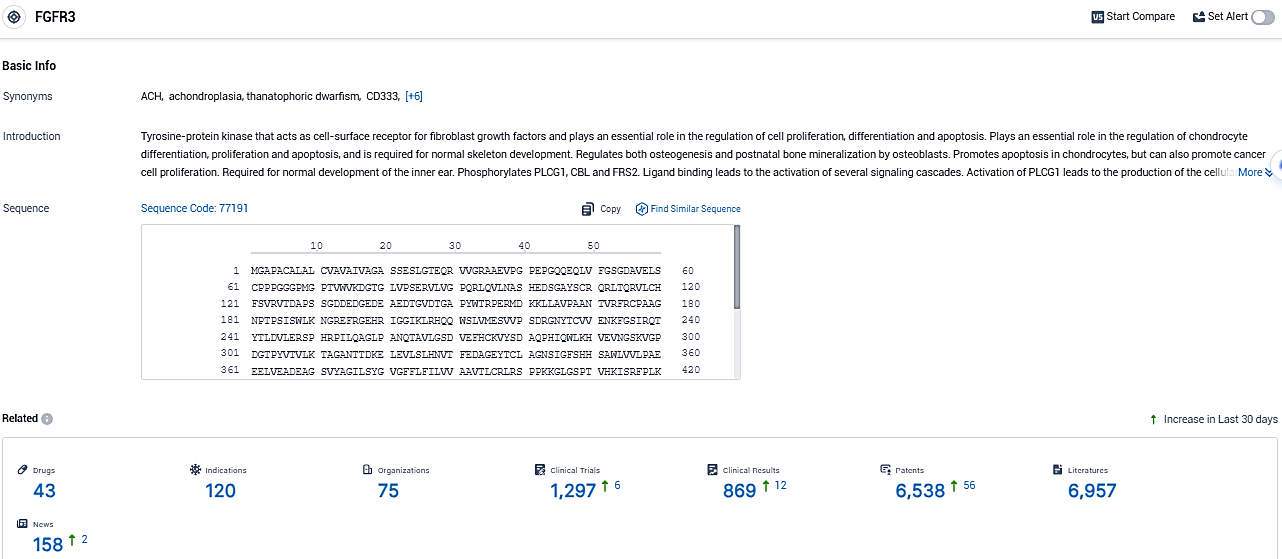
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 30, 2023, there are 43 investigational drugs for the FGFR3 target, including 120 indications, 75 R&D institutions involved, with related clinical trials reaching 1297, and as many as 6538 patents.
TYRA-300 is the Company's lead precision medicine program stemming from its in-house SNÅP platform. TYRA-300 is an investigational, oral, FGFR3-selective inhibitor currently in development for the treatment of cancer and skeletal dysplasias, including achondroplasia. In July 2023, TYRA-300 was granted Orphan Drug Designation for the treatment of achondroplasia from the FDA.