siRNA Therapeutics: From Molecular Mechanisms to Clinical Applications and Market Expansion
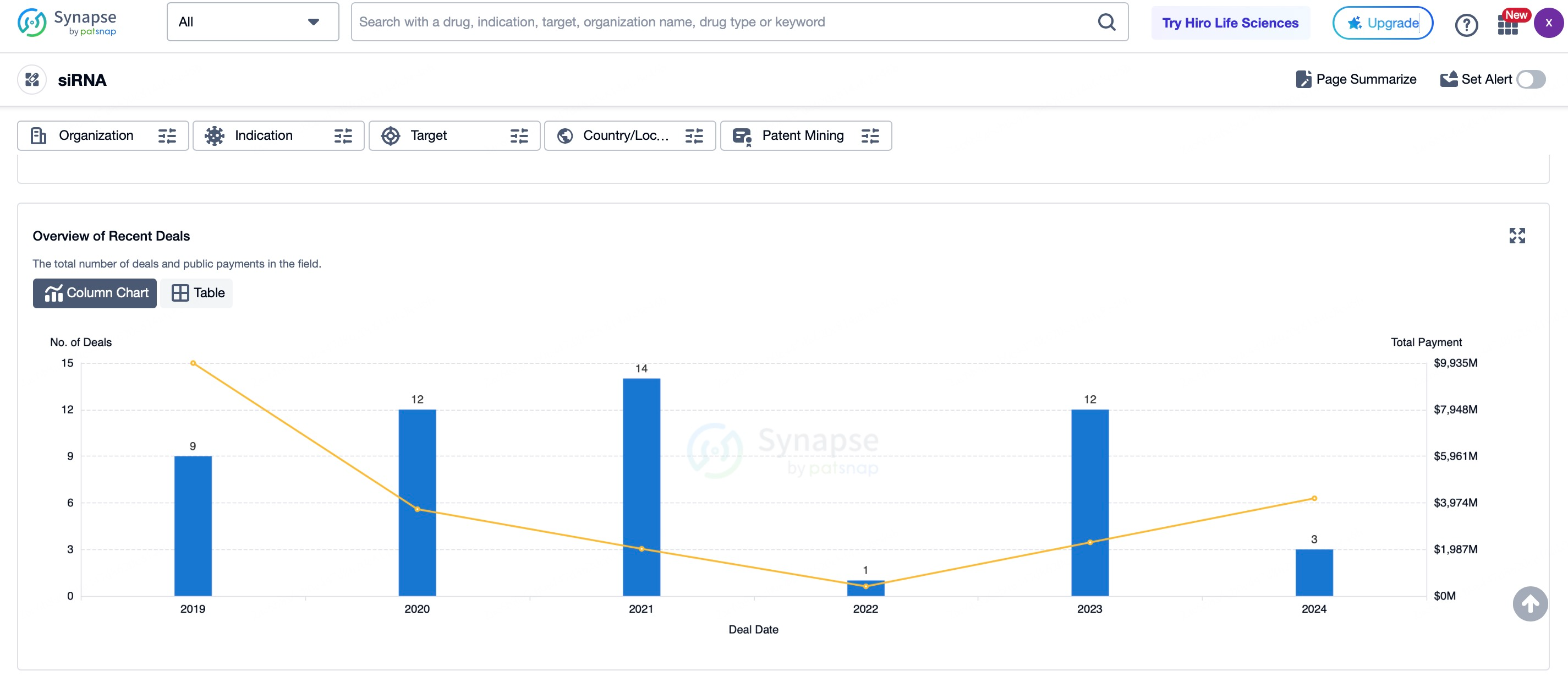
Currently, 6 siRNA drugs have been approved globally, including Patisiran, Inclisiran, and Amvuttra, which have demonstrated significant therapeutic effects in fields such as genetic diseases, cardiovascular diseases, and infectious diseases. Furthermore, according to statistics from Patsnap Synapse, at least 150 siRNA drugs are in clinical trials, covering a wide range of therapeutic areas. According to Maximize Market Research, the siRNA therapeutics market is expected to grow substantially from $12.7 billion in 2022 to $39.2 billion by 2029, with a compound annual growth rate (CAGR) of 25.3%.
siRNA drugs are synthesized chemically and typically consist of 21-23 nucleotides. They are composed of a guide strand (antisense strand) and a passenger strand (sense strand). Inside the cell, the generation of siRNA begins with long double-stranded RNA (dsRNA). These dsRNAs can originate from exogenous RNA viruses, transgenes, or transcription products of endogenous genes. Dicer, an RNA enzyme from the RNase III family, recognizes and cleaves long dsRNA to generate siRNA duplexes approximately 21-23 nucleotides in length. Dicer is responsible not only for cleaving dsRNA but also for processing and maturing the siRNA duplexes. The resulting siRNA duplexes are then loaded into the RNA-induced silencing complex (RISC). RISC is a multiprotein complex containing various proteins, with the most crucial component being the Argonaute protein, especially Ago2. Within RISC, the passenger strand (sense strand) is typically degraded or expelled, while the guide strand (antisense strand) is retained as the functional component of RISC.
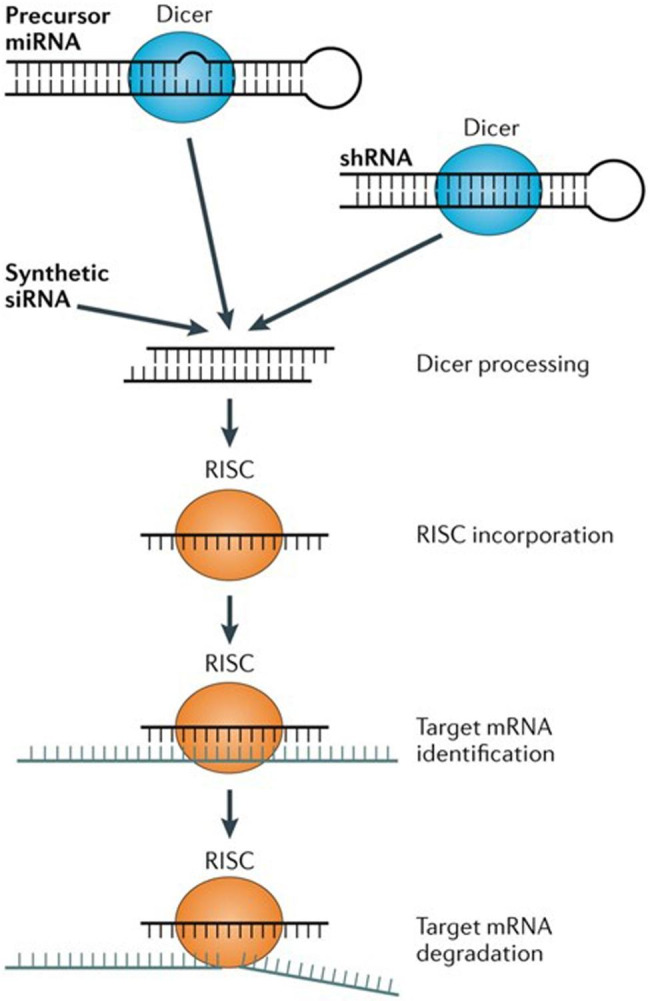
The RISC complex, loaded with the guide strand, searches for mRNA in the cytoplasm that is complementary to the guide strand. The guide strand binds to a specific region of the target mRNA through base pairing. This complementarity is typically perfect, ensuring high specificity of siRNA for the target mRNA. The recognition site for target mRNA is usually located in the 3' untranslated region (3' UTR), but it can also be in the coding region or other areas. RISC recognizes the target mRNA through precise base pairing, a process that depends on the complementarity between the guide strand and the mRNA.
Once the guide strand in the RISC complex successfully binds to the complementary sequence of the target mRNA, the Argonaute protein in RISC (especially Ago2) exerts its endonuclease activity and catalyzes the cleavage of the mRNA. Ago2 protein cleaves the mRNA at specific sites, causing mRNA fragmentation. The cleaved mRNA fragments are then further degraded, typically through other RNA degradation pathways within the cell, such as the action of exonucleases. This process completely disrupts the integrity of the mRNA, preventing it from being translated into protein, thereby inhibiting the expression of the target gene. Through mRNA degradation, siRNA effectively blocks the expression of the target gene, a process known as post-transcriptional gene silencing (PTGS). PTGS is widely present in both plants and animals and serves as an important mechanism for cells to regulate gene expression.
After completing one round of mRNA degradation, the RISC complex does not immediately become inactive but can be released and reused to search for new target mRNAs for further degradation. This recycling mechanism allows siRNA to continuously exert its effect within the cell, enhancing the efficiency and persistence of gene silencing. The levels of siRNA in the cell and the activity of RISC are in a dynamic equilibrium. Newly generated siRNA constantly replenishes RISC, while older siRNAs are gradually metabolized or degraded, ensuring the continuity and effectiveness of gene silencing.
Types of Modifications in siRNA Drugs and Their Effects
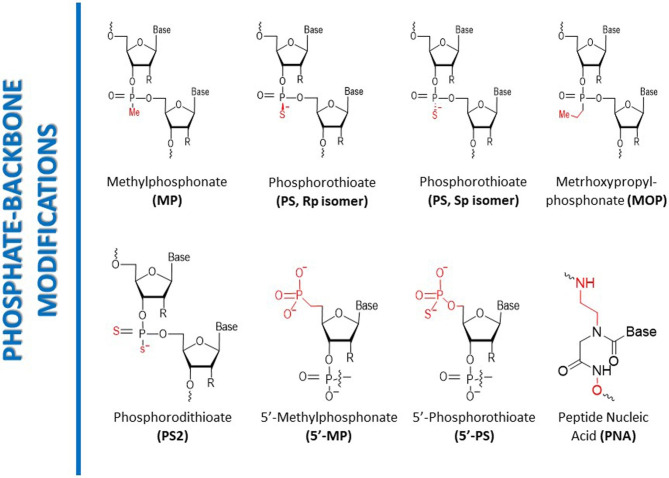
1. Phosphodiester Backbone Modifications
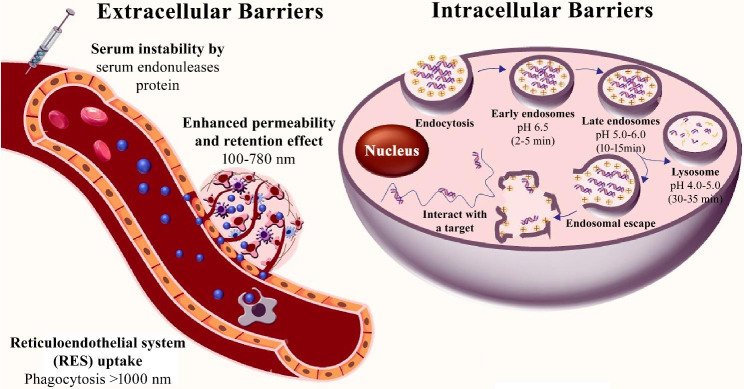
Phosphodiester backbone modifications in siRNA drugs primarily include thio-phosphodiester (PS) modifications and 5’-(E)-vinylphosphonate (5’-(E)-VP) modifications. Thio-phosphodiester modification replaces the phosphodiester bond in siRNA with a thio-phosphodiester bond, significantly enhancing the stability of siRNA and its resistance to nucleases. This modification also increases the lipophilicity of siRNA, facilitating its penetration through the cell membrane. However, excessive PS modification may reduce the binding affinity of siRNA to the target mRNA, potentially affecting the efficiency of gene silencing. The 5’-(E)-VP modification is a novel phosphate mimic, where a vinylphosphonate group is introduced at the 5’ end of siRNA to enhance its stability in vivo. This modification not only makes siRNA resistant to exonuclease degradation and improves its silencing effect in vivo, but it also increases the binding affinity of siRNA to Ago2 protein, improving its loading efficiency into RISC.
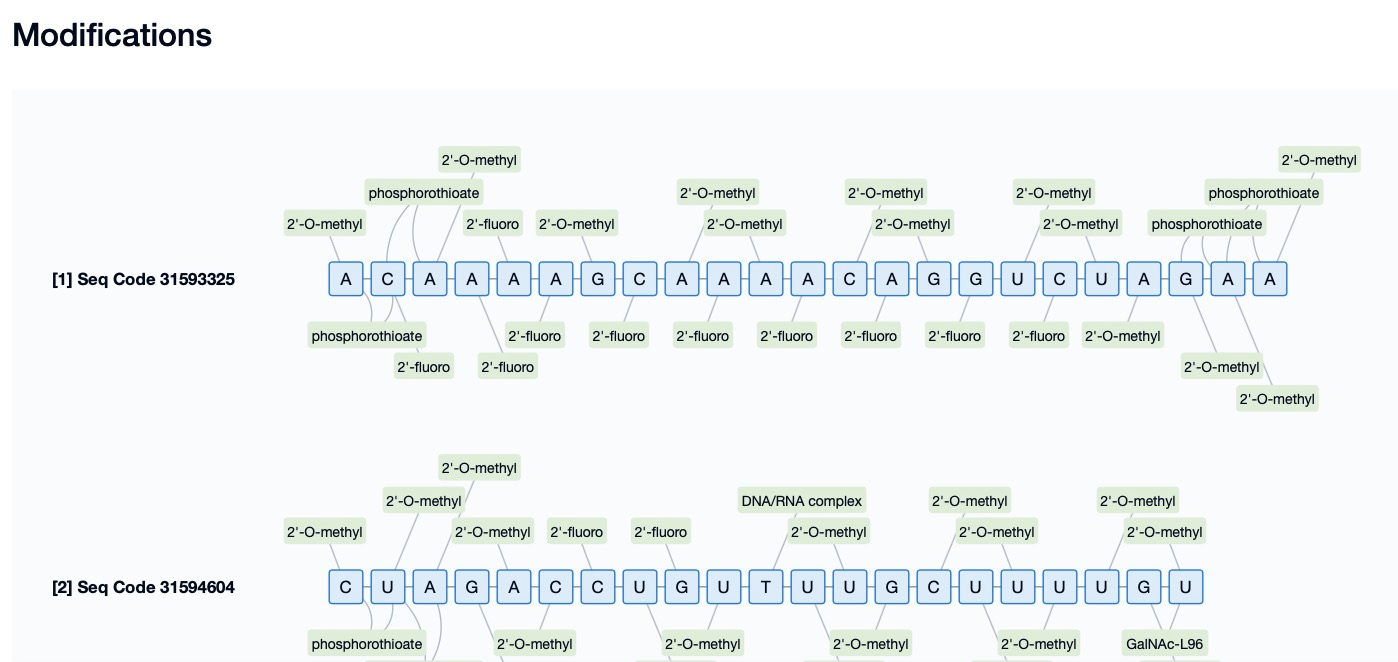
For example, Inclisiran, developed by Alnylam Pharmaceuticals, is an siRNA drug for treating hypercholesterolemia that incorporates 5’-(E)-VP modification. This modification significantly improves the in vivo stability and gene-silencing efficacy of Inclisiran. The 5’-(E)-VP modification enables Inclisiran to resist degradation by exonucleases more effectively, maintaining its activity in the body for a longer duration. Additionally, the 5’-(E)-VP modification enhances the binding affinity of Inclisiran to the Ago2 protein, increasing its loading efficiency into RISC and further boosting its gene-silencing efficacy. The successful development and clinical application of Inclisiran demonstrate the significant potential of 5’-(E)-VP modification in enhancing the performance of siRNA drugs.
2. Ribose Modifications
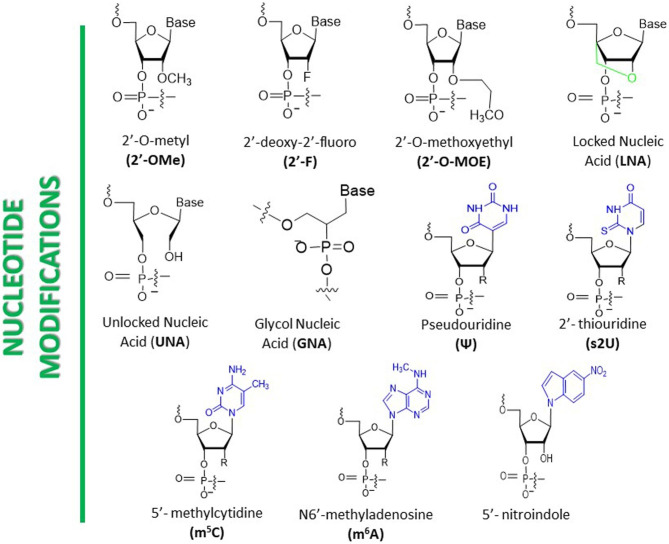
Ribose modifications are effective strategies for improving siRNA stability and reducing immunogenicity. Common ribose modifications include 2’-O-methyl (2’-OMe), 2’-O-methoxyethyl (2’-MOE), and 2’-fluoro (2’-F) modifications. The 2’-OMe modification introduces a methyl group at the 2’ position of the ribose, enhancing siRNA stability and nuclease resistance while reducing immunogenicity. This modification has minimal impact on the base-pairing ability of siRNA and does not significantly affect its gene-silencing efficacy. The 2’-MOE modification introduces a methoxyethyl group at the 2’ position of the ribose, further improving siRNA stability and nuclease resistance, significantly reducing immunogenicity, but potentially slightly decreasing its gene-silencing efficacy. The 2’-F modification introduces a fluorine atom at the 2’ position of the ribose, enhancing siRNA stability and nuclease resistance, and reducing immunogenicity, though it may slightly reduce gene-silencing efficacy.
For example, Patisiran, developed by Alnylam Pharmaceuticals, is an siRNA drug for treating hereditary transthyretin amyloidosis (hATTR) that incorporates 2’-OMe and 2’-F modifications. By introducing 2’-OMe and 2’-F modifications at specific positions on the sense and antisense strands of siRNA, Patisiran significantly improves its stability and nuclease resistance while reducing immunogenicity. These modifications enable Patisiran to maintain its activity in vivo for a longer duration, effectively degrading the target mRNA and reducing the accumulation of abnormal proteins, thereby alleviating disease symptoms. The successful development and clinical application of Patisiran demonstrate the significant potential of 2’-OMe and 2’-F modifications in enhancing the performance of siRNA drugs.
3. Base Modifications
Base modifications enhance the stability and nuclease resistance of siRNA by altering its nucleotide bases. Common base modifications include locked nucleic acid (LNA) and glycol nucleic acid (GNA). LNA is a unique bicyclic nucleotide derivative containing one or more 2’-O, 4’-C-methylene-β-D-ribofuranose monomers. The methylene bridge between the 2’ oxygen and 4’ carbon atoms of the ribose locks the sugar ring into a rigid bicyclic structure, significantly reducing the flexibility of the ribose and enhancing the local stability of the phosphate backbone. LNA modification markedly improves the thermal stability of siRNA, making it more stable in vivo, while also increasing its binding affinity to the target mRNA, thereby enhancing gene-silencing efficacy. GNA, on the other hand, features an acyclic three-carbon propylene glycol (1,2-propanediol) backbone, replacing the (deoxy)ribose of DNA and RNA to form the simplest chemically stable nucleic acid structure. GNA modification enhances the binding affinity of siRNA, improves its gene-silencing efficacy, reduces immunogenicity, and minimizes immune responses. These base modifications not only improve the stability and degradation resistance of siRNA but also significantly enhance its gene-silencing efficacy, thereby improving the safety and therapeutic effectiveness of siRNA drugs.
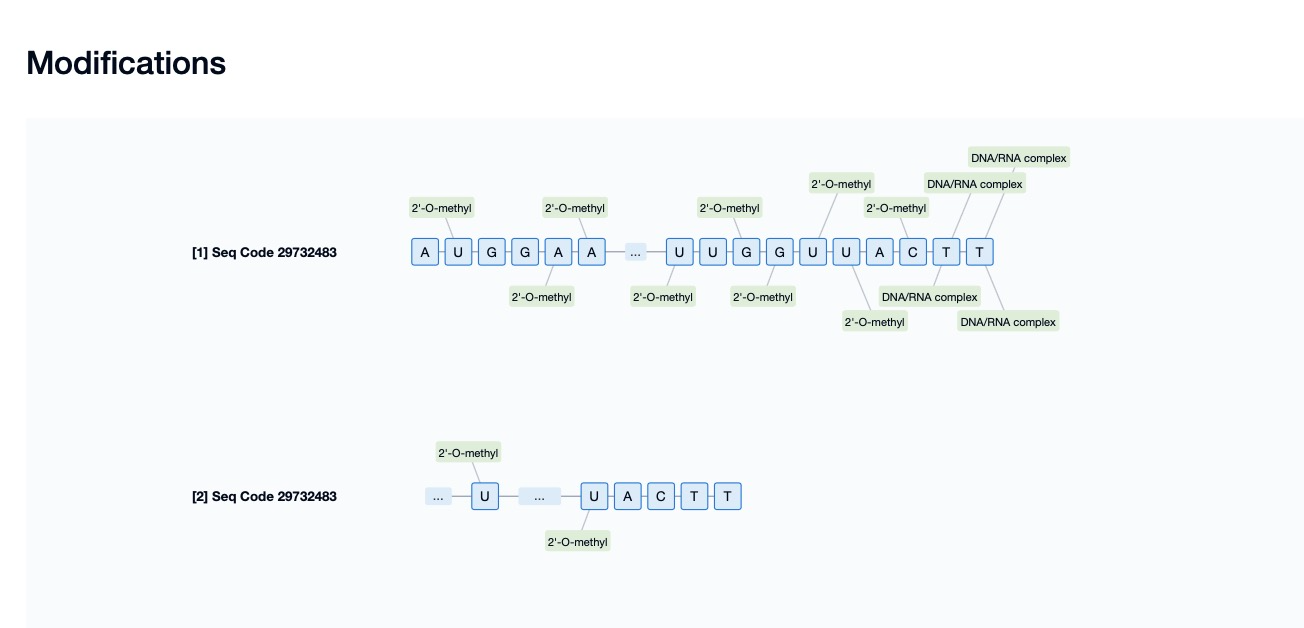
For example, Givosiran, developed by Alnylam Pharmaceuticals, is an siRNA drug for treating acute hepatic porphyria (AHP) that incorporates LNA modification. By introducing LNA modifications at specific positions on the antisense strand of siRNA, Givosiran significantly improves its thermal stability and nuclease resistance while enhancing its binding affinity to the target mRNA, thereby boosting gene-silencing efficacy. This modification enables Givosiran to maintain its activity in vivo for a longer duration, effectively degrading the target mRNA and reducing the accumulation of porphyrin precursors, thereby alleviating disease symptoms.
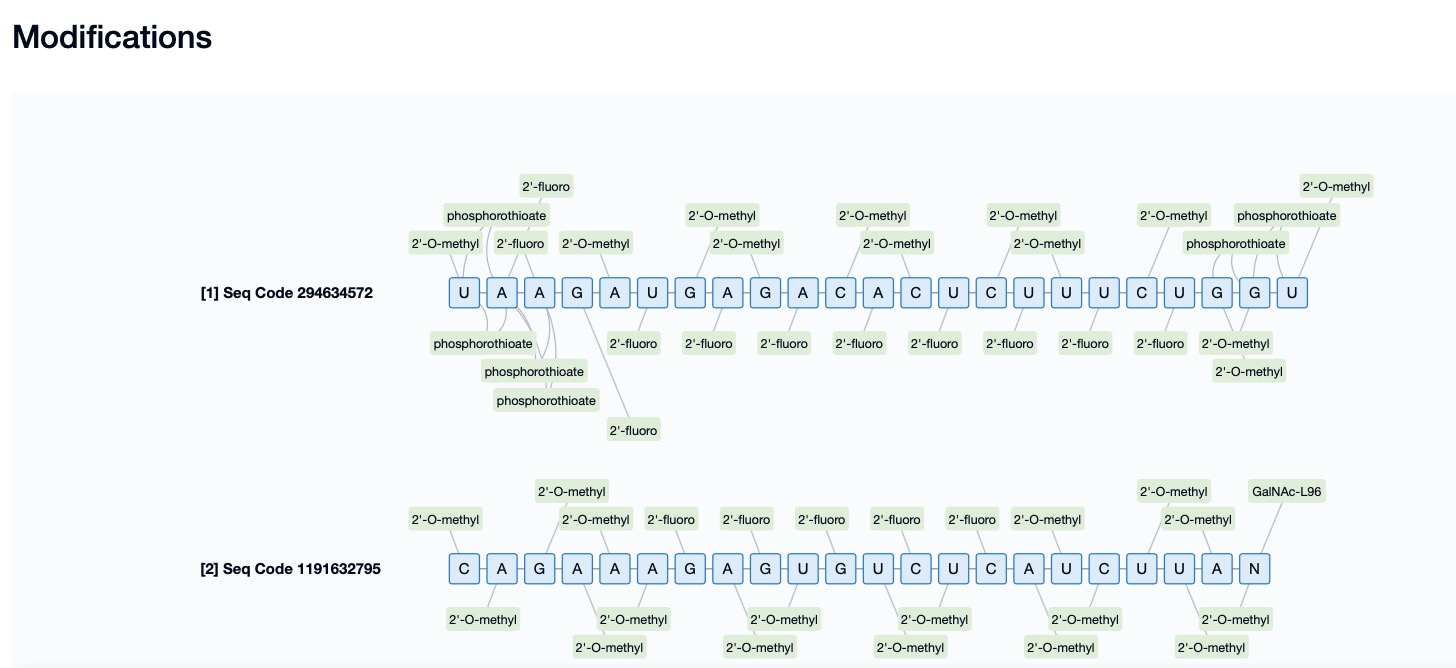
4. Terminal Modifications
Terminal modifications enhance the stability and nuclease resistance of siRNA by introducing specific chemical groups at the 5’ or 3’ ends. Common 5’ terminal modifications include 5’-phosphorylation and 5’-methylphosphonate (5’-MP) modifications. The 5’-phosphorylation modification involves introducing a phosphate group at the 5’ end of siRNA, which enhances the binding affinity of siRNA to the Ago2 protein, improves its loading efficiency into the RNA-induced silencing complex (RISC), and thereby enhances gene-silencing efficacy. The 5’-MP modification introduces a methylphosphonate group at the 5’ end, which not only improves siRNA stability but also enhances its resistance to nucleases. Common 3’ terminal modifications include 3’-inverted deoxynucleotide (3’-ddN), which can reduce the immunogenicity of siRNA, minimize immune responses, and improve gene-silencing efficacy. These terminal modifications not only enhance the stability and degradation resistance of siRNA but also significantly improve its gene-silencing efficacy, thereby increasing the safety and therapeutic effectiveness of siRNA drugs.
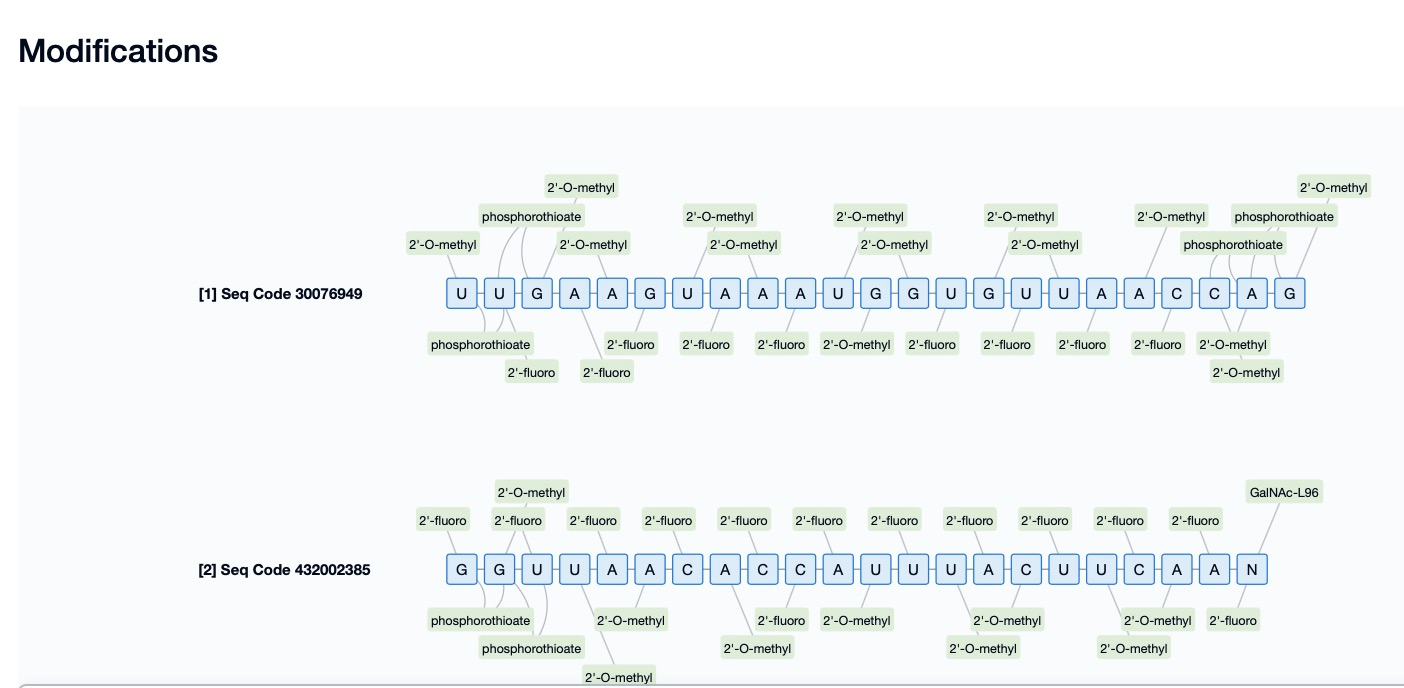
Fitusiran, developed by Alnylam Pharmaceuticals, is an siRNA drug used to treat hemophilia A and B. By targeting the mRNA of antithrombin (AT), Fitusiran reduces its expression in the liver, thereby increasing the levels of clotting factors in the blood and reducing bleeding episodes. Fitusiran incorporates 5’-phosphorylation modification, which introduces a phosphate group at the 5’ end of siRNA, significantly enhancing its binding affinity to the Ago2 protein. This modification enables siRNA to bind more effectively to the Ago2 protein after entering cells, forming the RNA-induced silencing complex (RISC), which specifically recognizes and degrades the mRNA of antithrombin, reducing its expression. The 5’-phosphorylation modification not only improves the gene-silencing efficacy of Fitusiran but also enhances its in vivo stability and degradation resistance, reduces immunogenicity, and minimizes potential side effects.
Summary
As an emerging therapeutic technology, siRNA drugs have demonstrated significant therapeutic effects in areas such as genetic disorders, cardiovascular diseases, and infectious diseases. Through the aforementioned modification strategies, the stability and specificity of siRNA drugs have been significantly improved, while immunogenicity and off-target effects have been reduced, enhancing their bioavailability and therapeutic efficacy in vivo. The development of these modification technologies has provided a solid foundation for the application of siRNA drugs in the treatment of various diseases and has driven the rapid growth of the siRNA therapeutics market.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference
- 1.Ali Zaidi SS, Fatima F, Ali Zaidi SA, Zhou D, Deng W, Liu S. Engineering siRNA therapeutics: challenges and strategies. J Nanobiotechnology. 2023 Oct 18;21(1):381. doi: 10.1186/s12951-023-02147-z. PMID: 37848888; PMCID: PMC10583313.
- 2.Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017 Mar;16(3):181-202. doi: 10.1038/nrd.2016.199. Epub 2016 Nov 3. Erratum in: Nat Rev Drug Discov. 2017 Jun;16(6):440. doi: 10.1038/nrd.2017.86. PMID: 27807347; PMCID: PMC5700751.