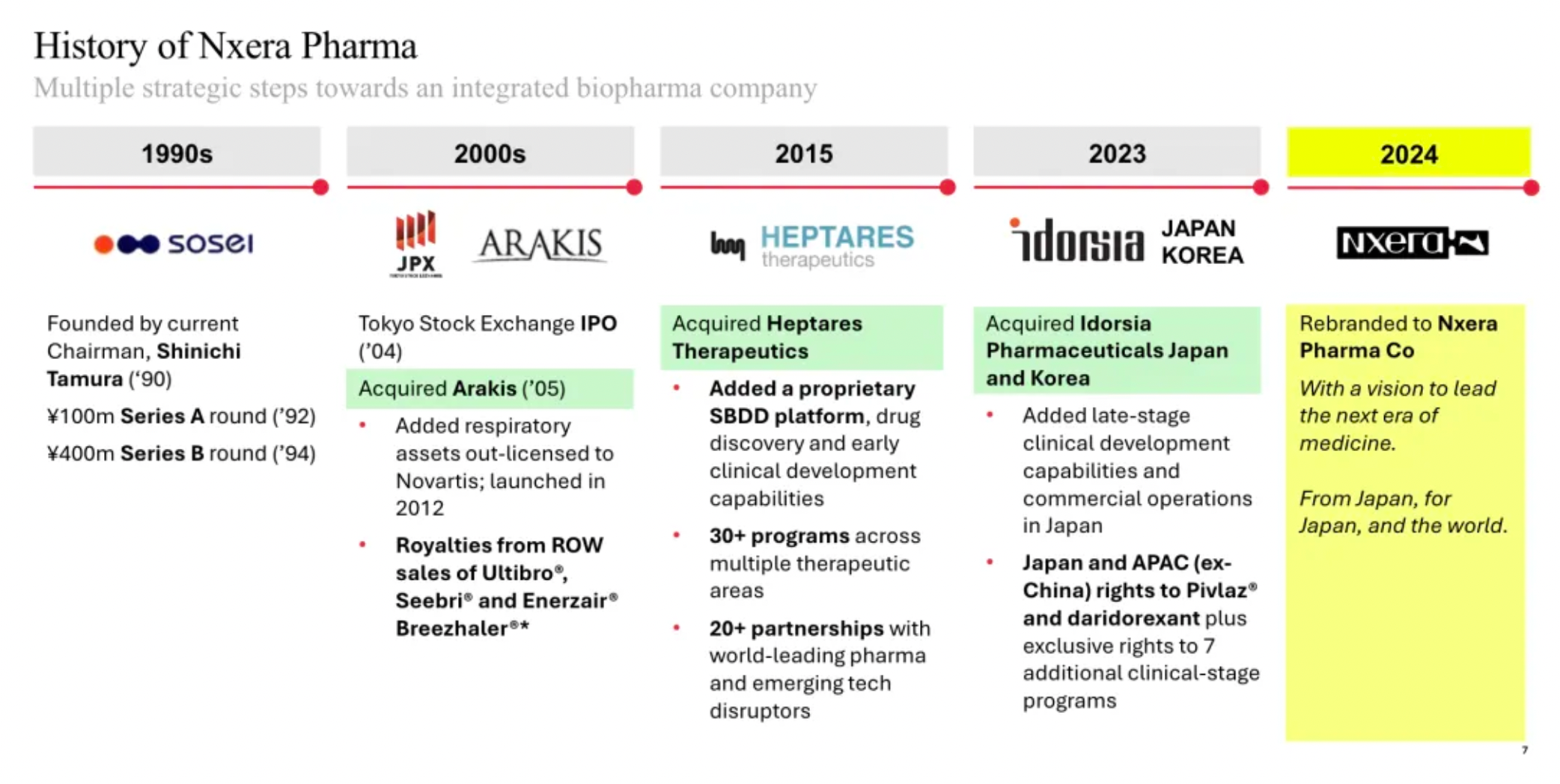
Sosei Heptares has been renamed Nxera Pharma, with updates made to its corporate structure
On March 27th, Sosei Group Corporation of Japan announced that during the 34th Annual General Meeting held in Tokyo on the same day, followed by a subsequent board meeting, the reelection of the company’s board members and executives was approved. Additionally, the Annual General Meeting approved a "partial amendment to the corporate charter (change of company name)." With this approval, the company will be renamed Nxera Pharma Corporation starting April 1, 2024.
The name "Nxera" is derived from the words "Next" and "Era," intended to express the company’s determination to be a leader in a new era of science and medical advancement, as stated in the press release. The mission of the new company is to be "a technology-driven pharmaceutical enterprise devoted to breaking the status quo and providing better treatment options for patients across multiple therapeutic areas."
Business Overview
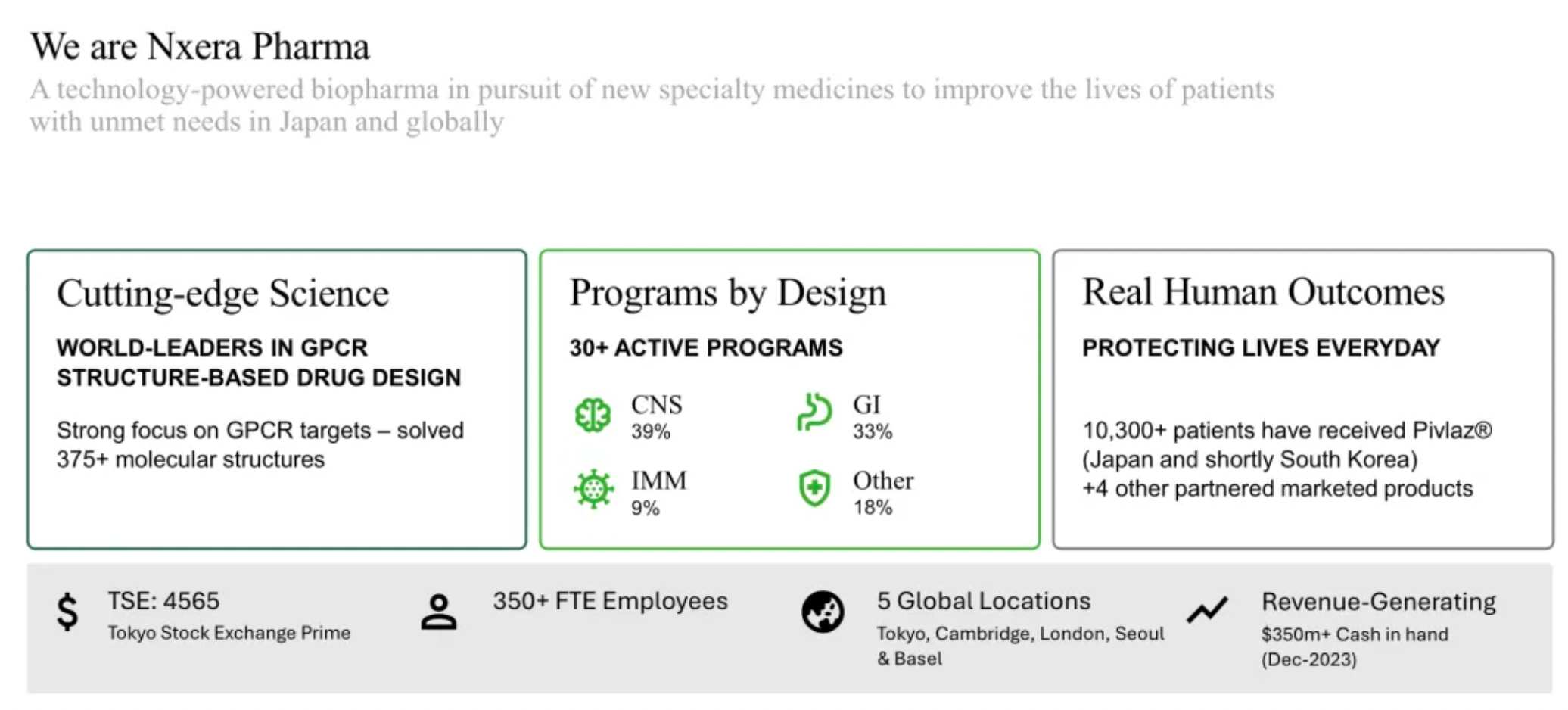
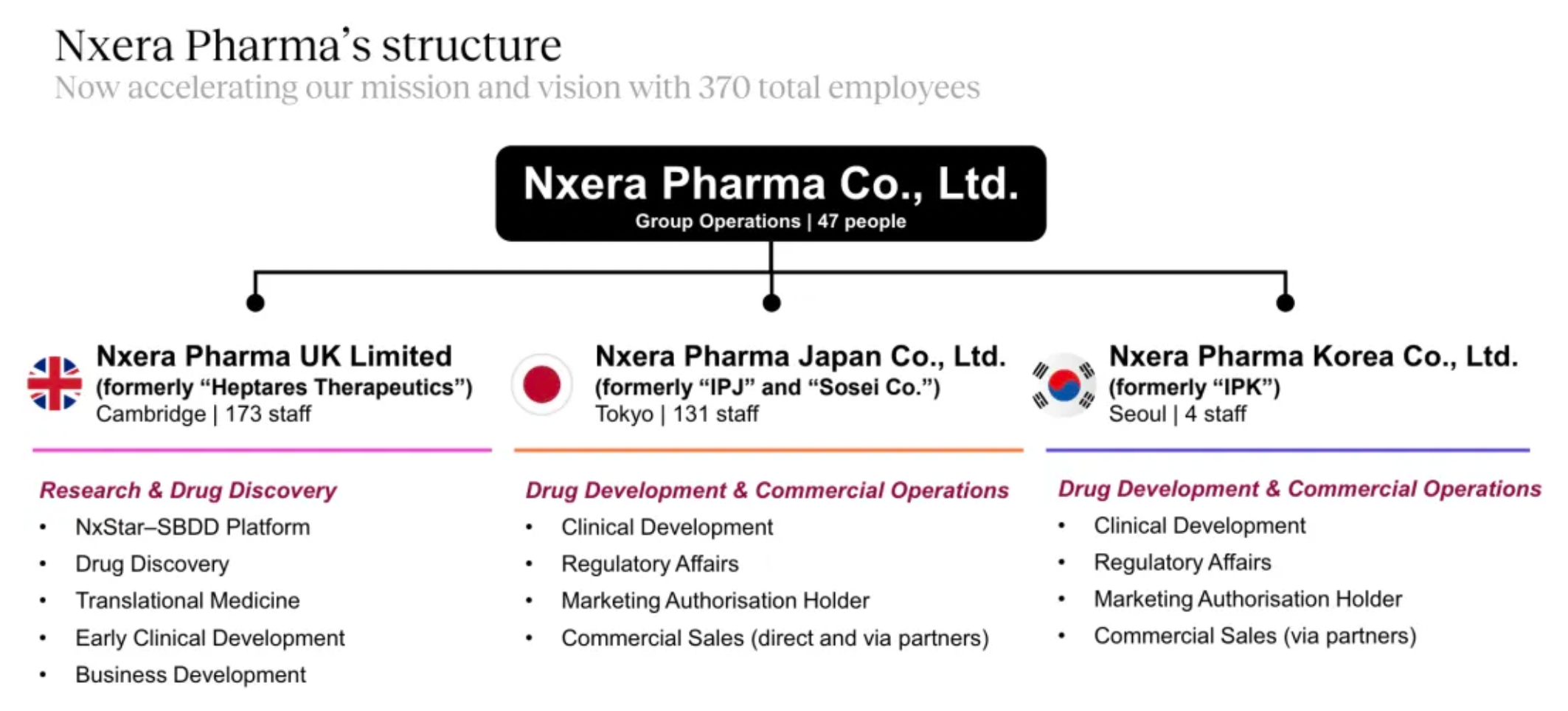
Drug Discovery + Drug Development and Commercialization: The European site is responsible for basic research and early-stage drug discovery, while the sites in Japan and South Korea primarily handle the clinical development of drugs.
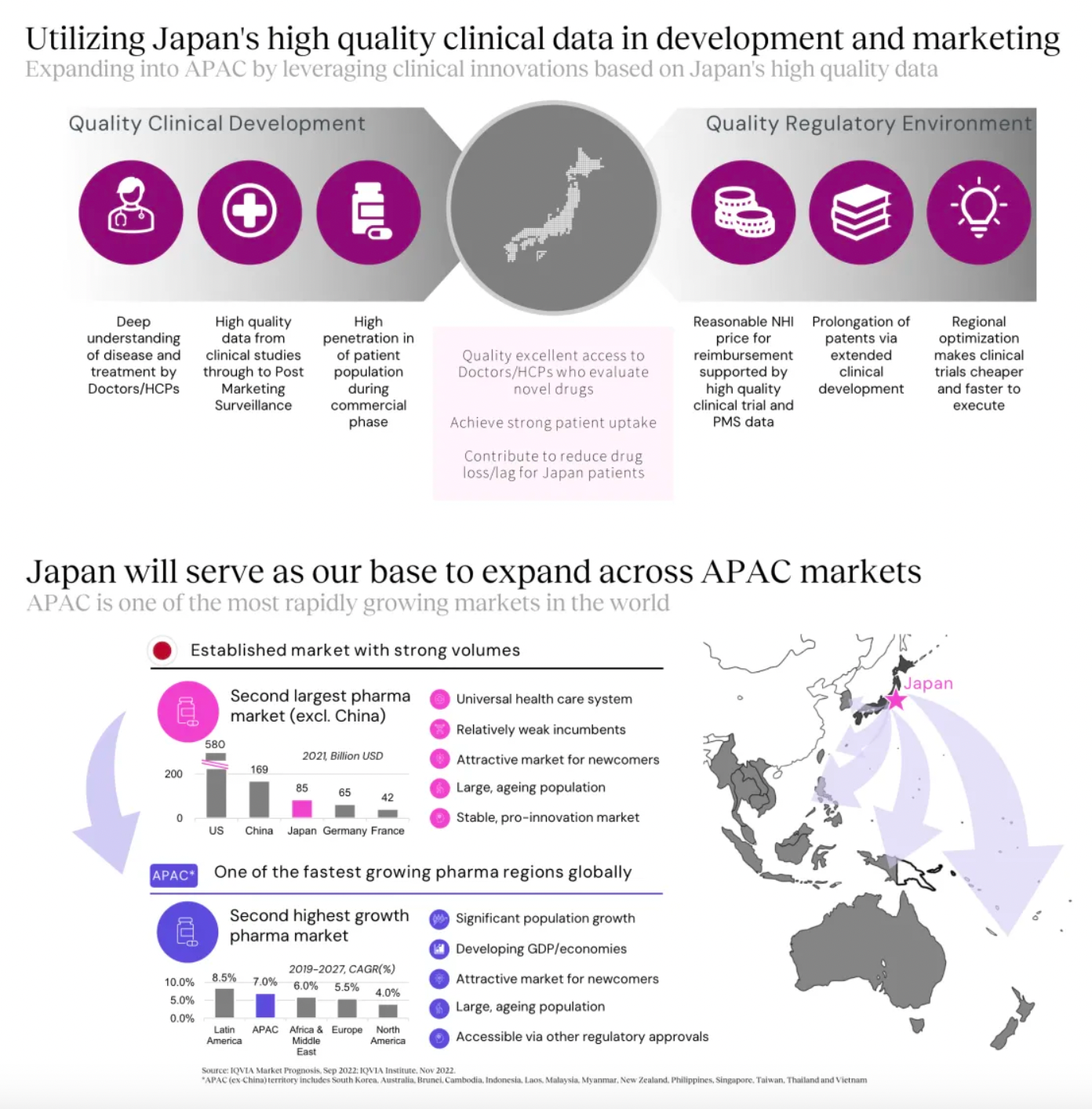
Based on high-quality clinical data from Japan, we are conducting clinical development for our internal projects. Simultaneously, the company is also bringing some U.S.-approved drugs to Japan for Phase III clinical studies, using this as a platform to reach the Asian market.
Financial Performance
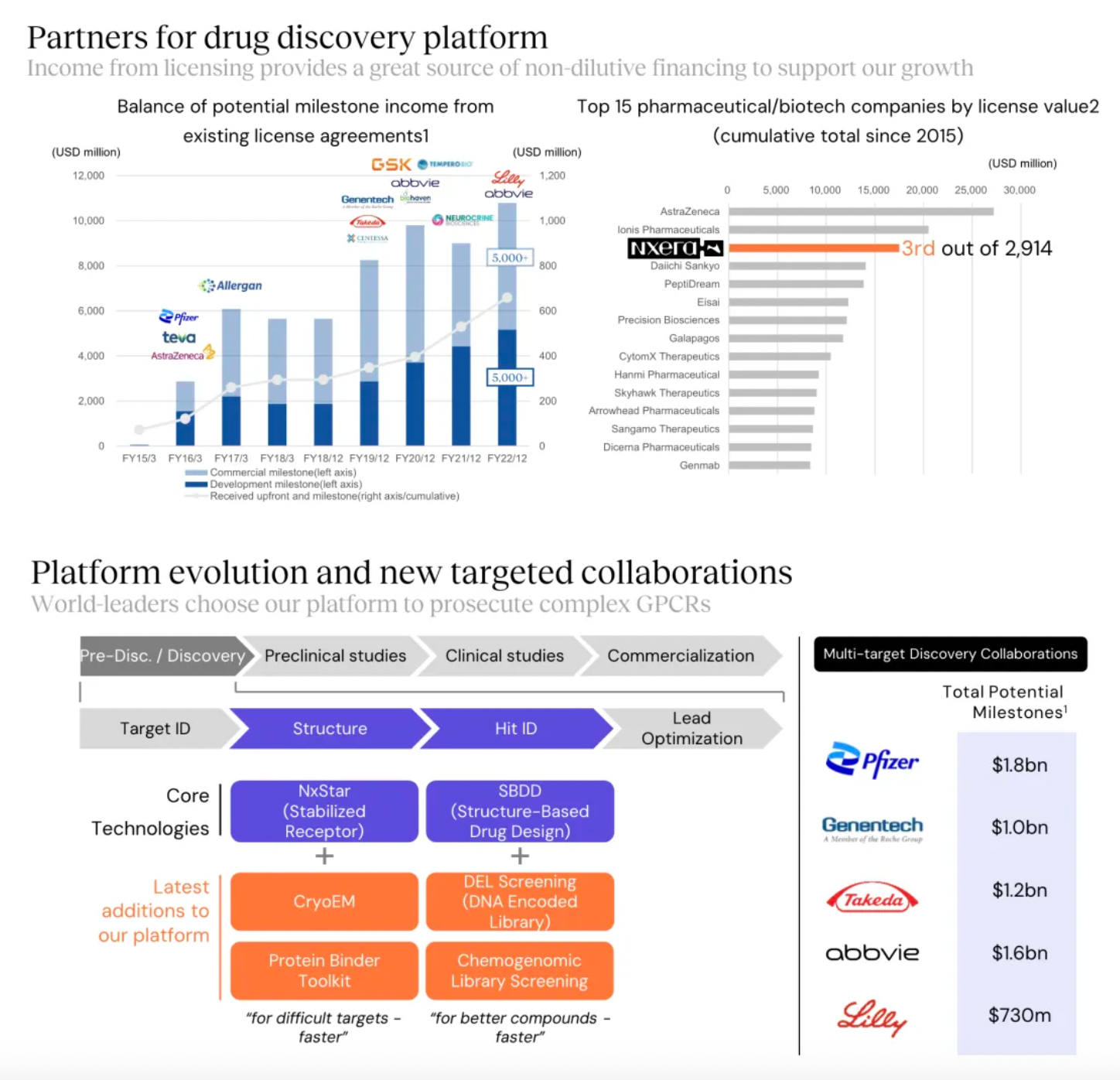
Overall, the financial data for 2023 shows a decline compared to both 2021 and 2022. It is worth mentioning that in 2023, there were no new out-licensing deals in GPCR drug discovery, the area where the Sosei Hectares team excels. Regarding milestone payment revenues, there was a significant reduction in 2023, partly due to the failure of clinical studies for several GLP-1 small molecule agonists developed in collaboration with Pfizer.

R&D pipeline
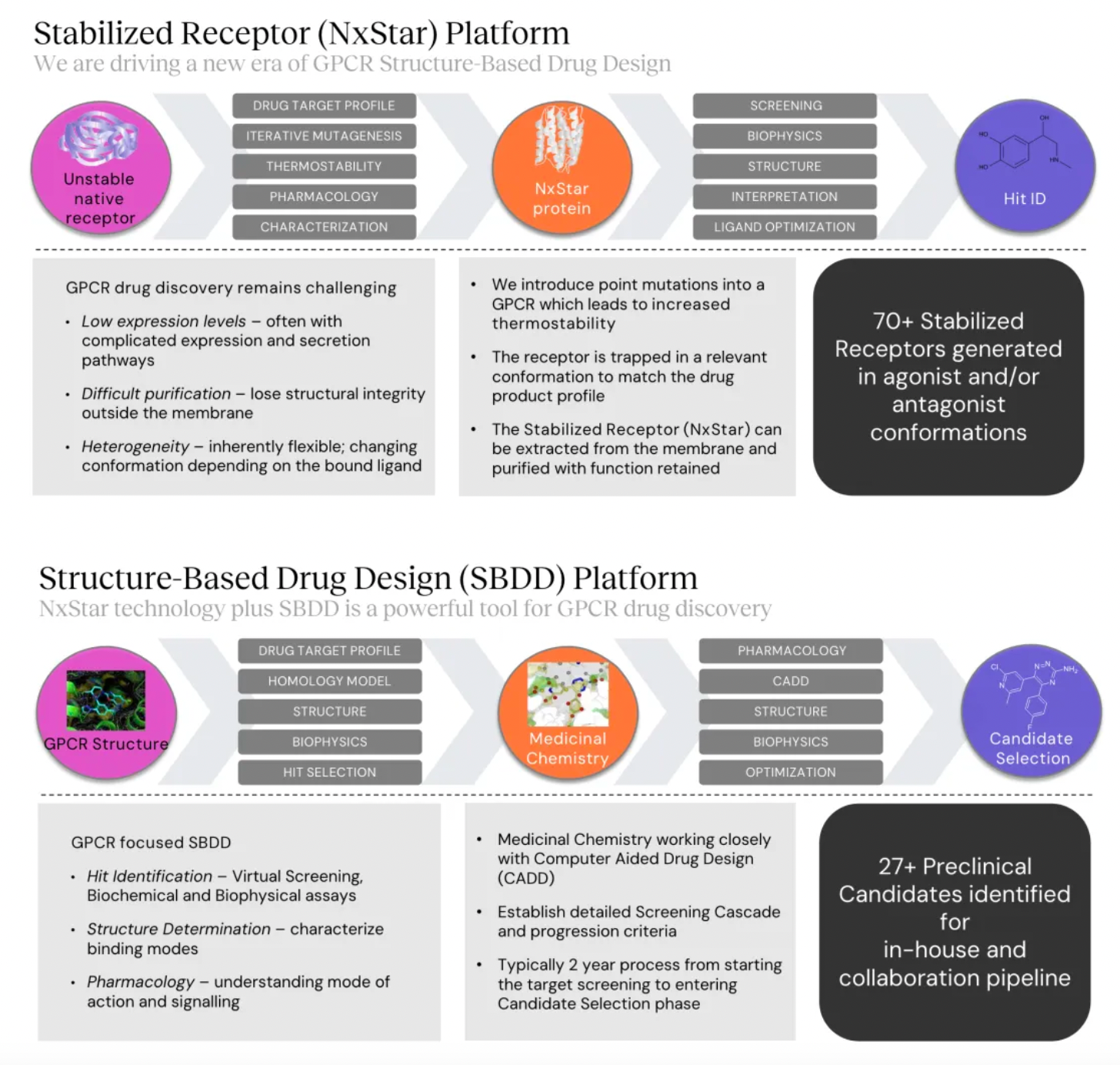
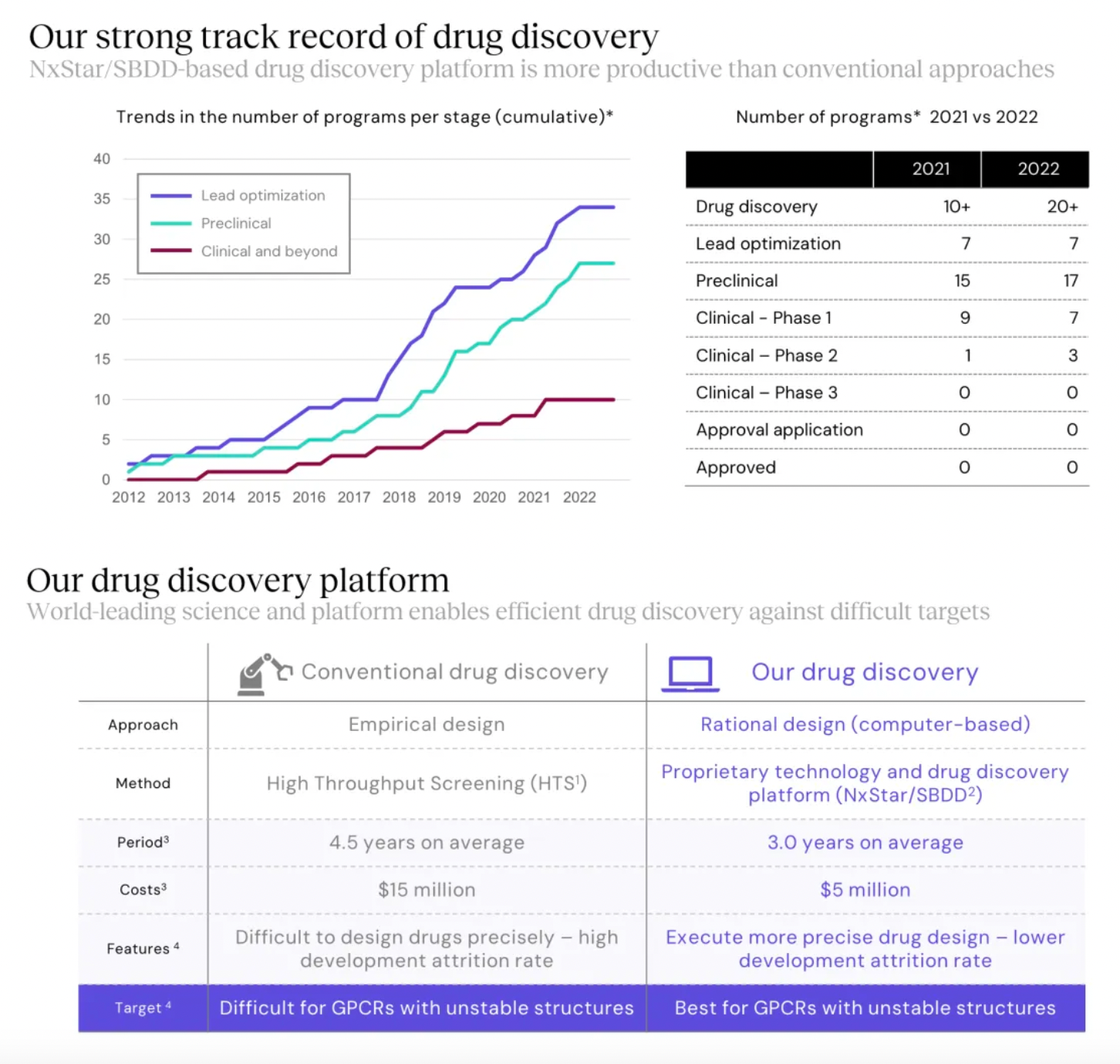
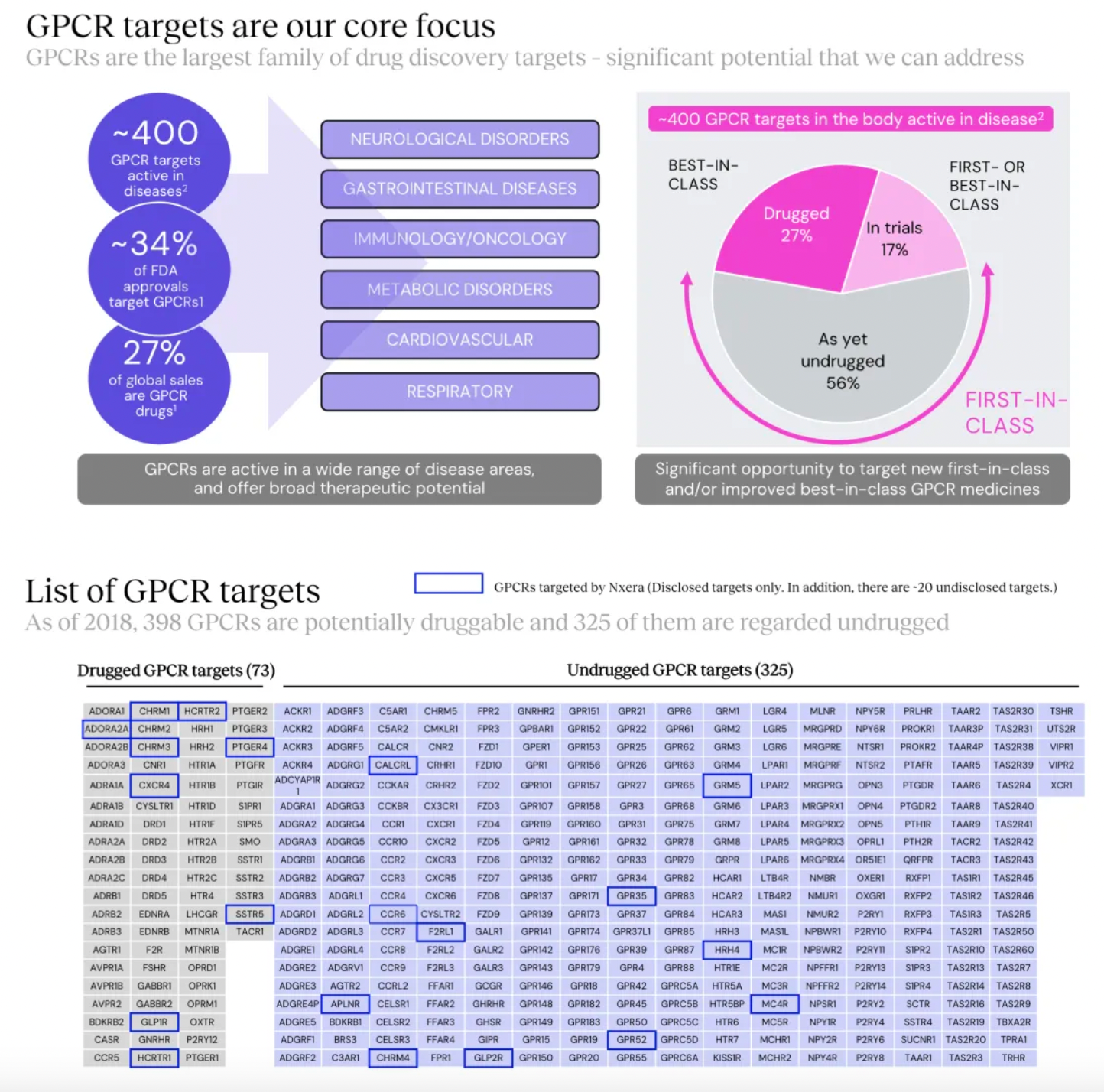
Focusing on potential GPCR targets, the Nxera team has continuously excelled in driving the development of innovative small molecule drugs using a structure-based drug discovery approach.
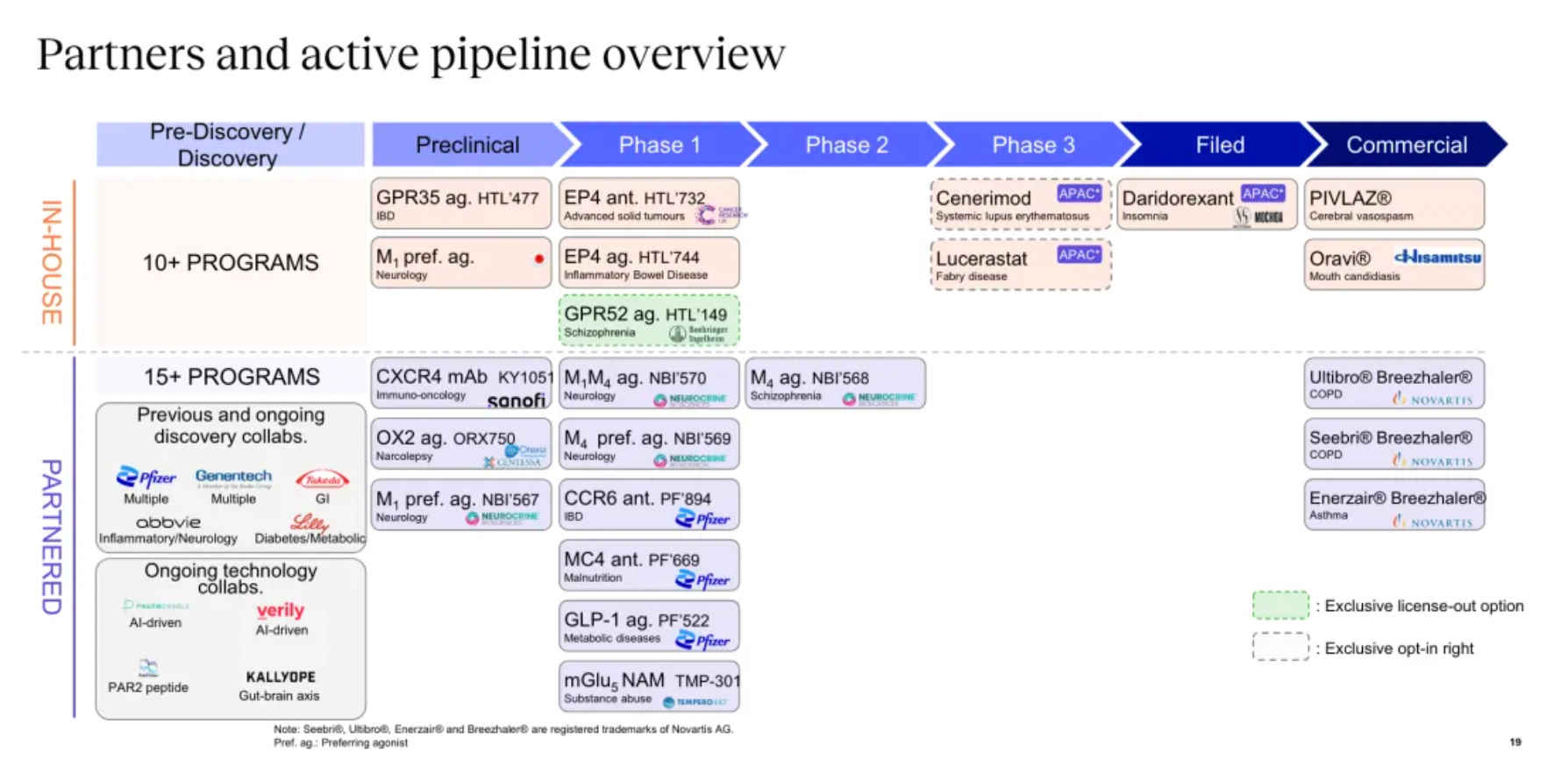
Targeting GPR52 for the development of clinical candidates in schizophrenia is the only new transfer project of the Nxera team after 2022.
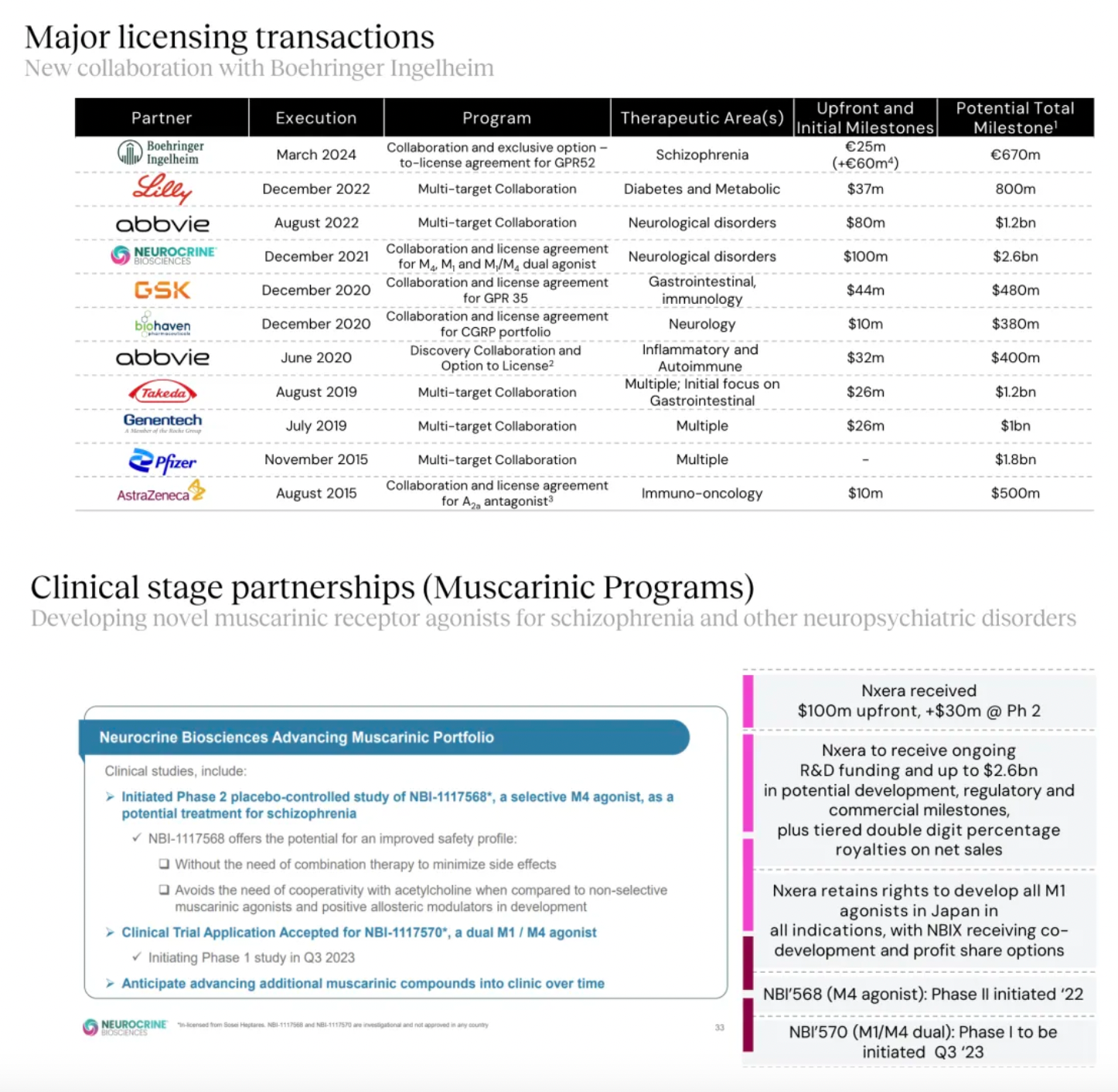
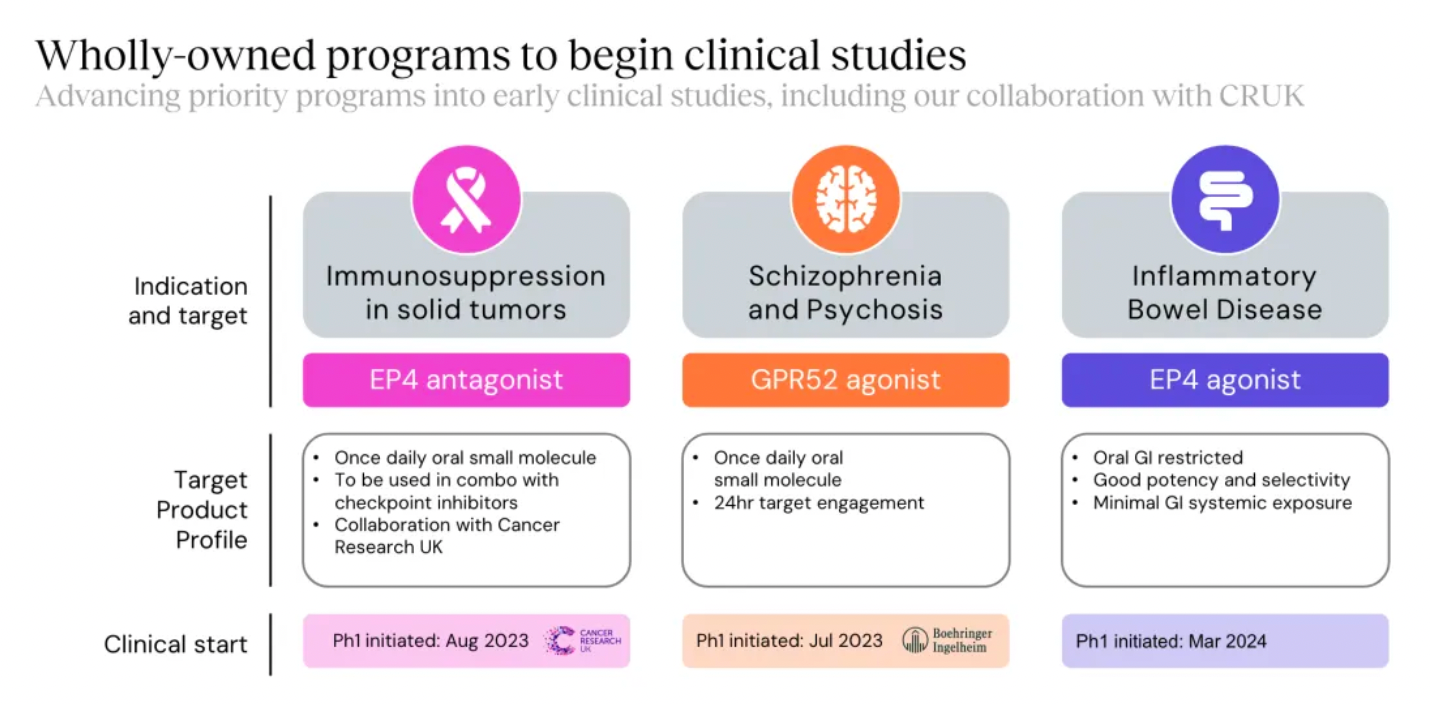
New projects advanced into clinical research and development include the EP4 antagonist, EP4 agonist project, and the GPR52 agonist project.
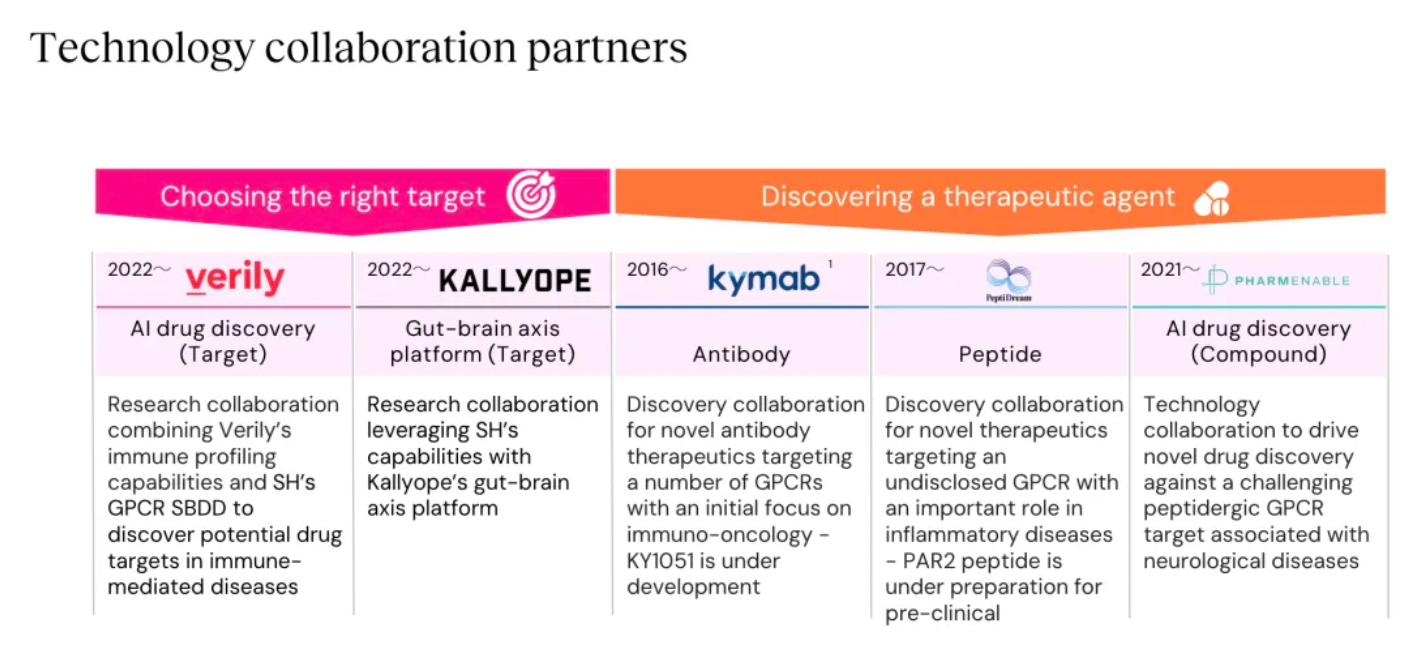
Stable GPCR Research and Development Platform
Representative drugs
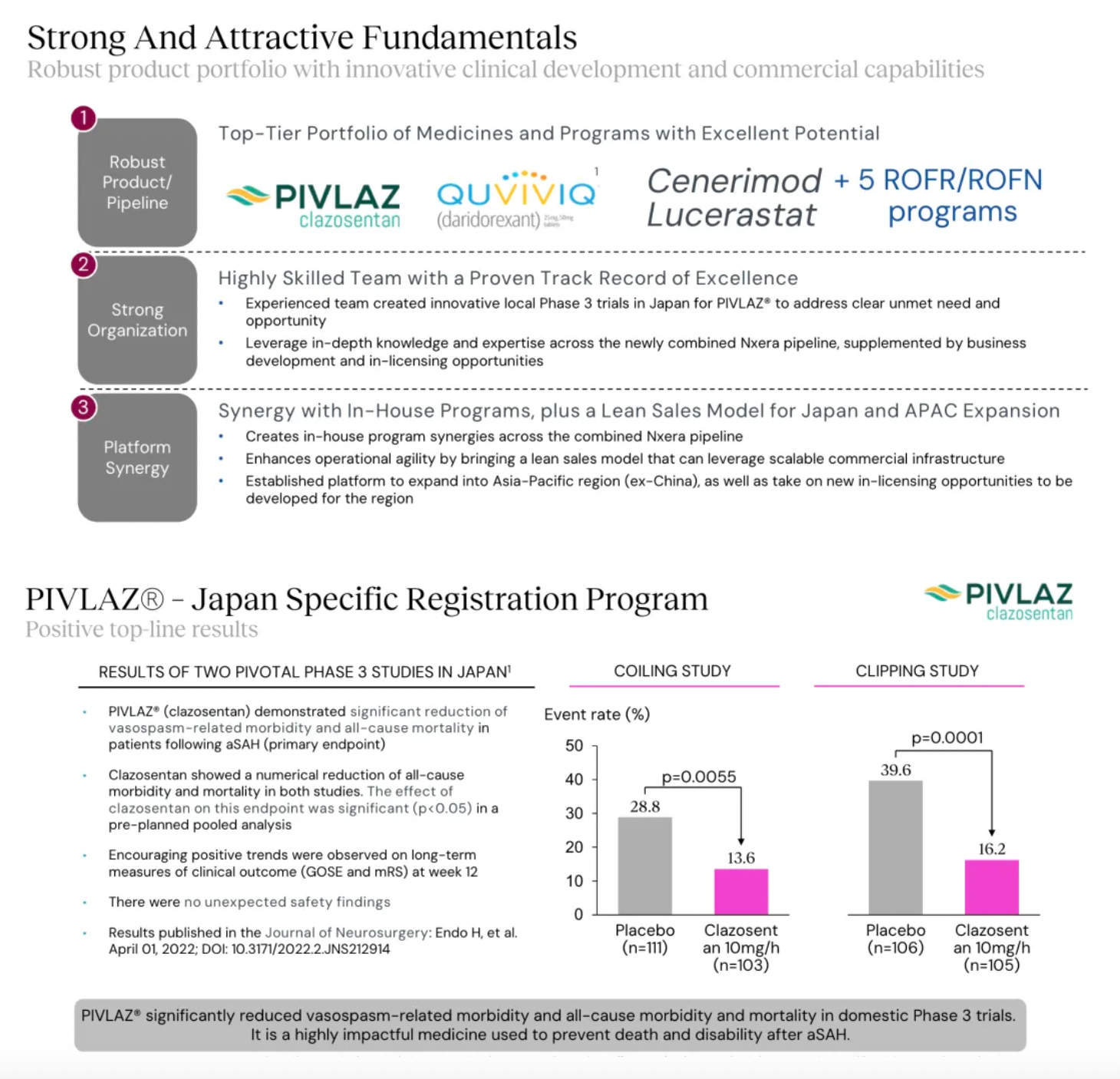
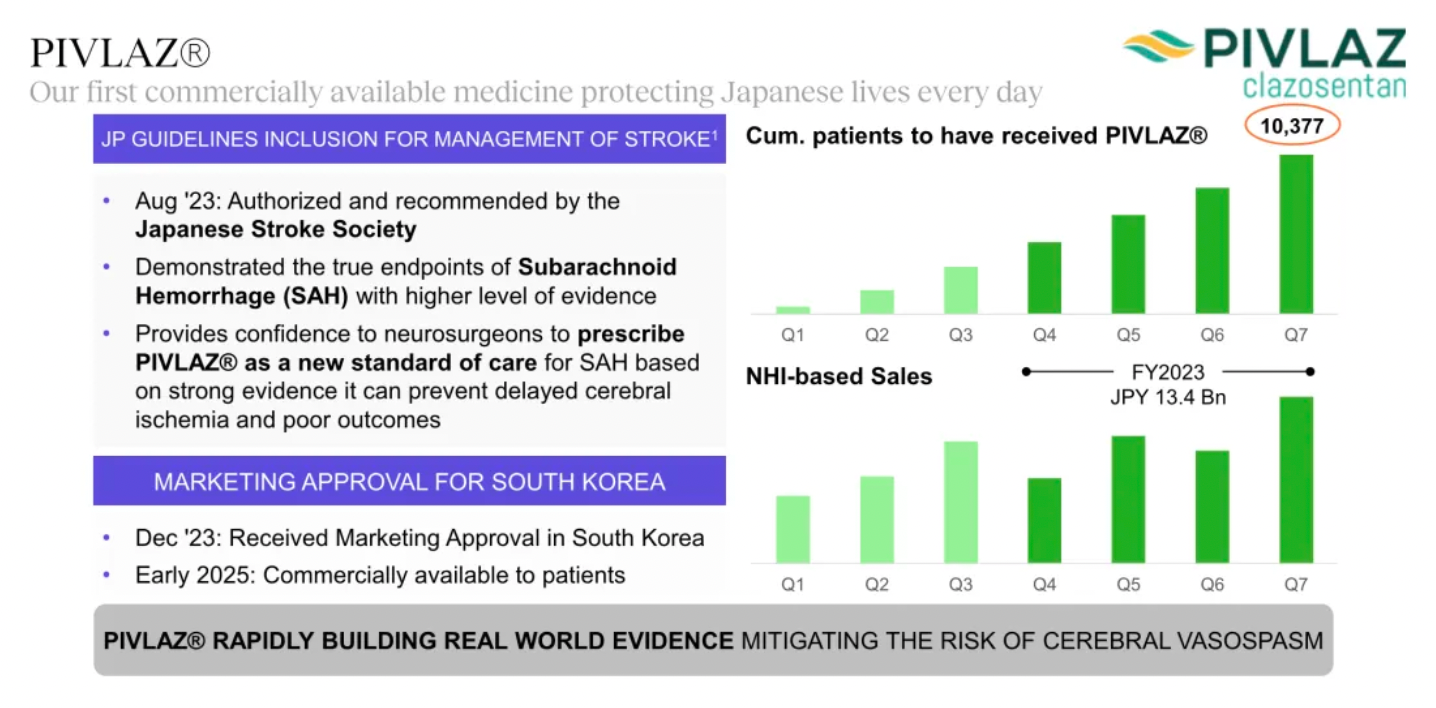
The company's first commercially marketed drug, PIVLAZ® (a selective ETA receptor antagonist), has made a significant impact on stroke treatment in Japan and accumulated real-world evidence. PIVLAZ® has been recommended by the Japanese Stroke Society and proven to effectively treat subarachnoid hemorrhage (SAH). It can prevent delayed cerebral ischemia and poor outcomes with high-level evidence, thus enhancing the confidence of neurosurgeons in prescribing this medication.
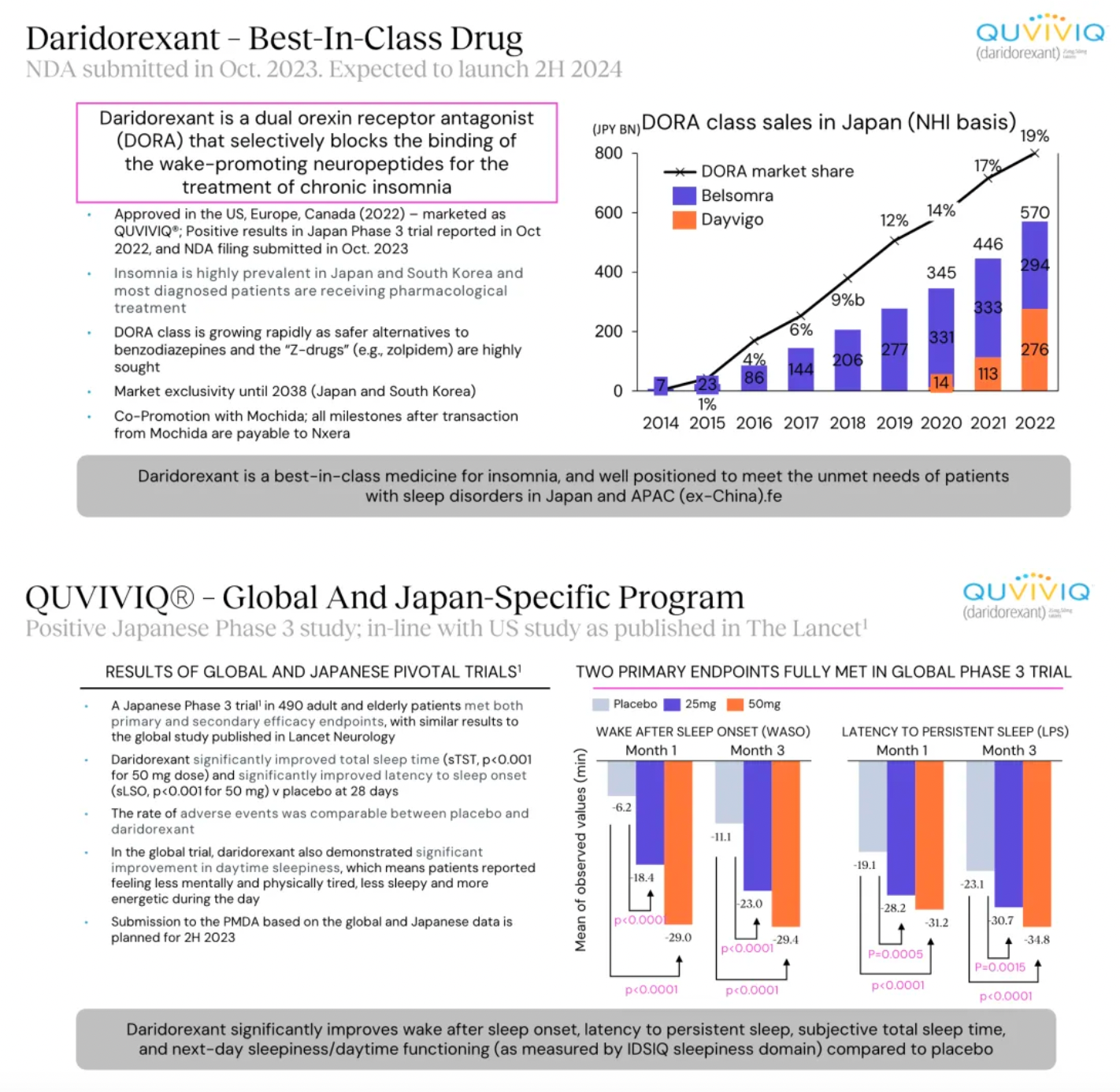
Daridorexant is a dual orexin receptor antagonist that blocks the binding and activity of the wakefulness-promoting neuropeptide orexin. At the end of 2023, a New Drug Application for daridorexant was submitted in Japan for the treatment of insomnia, with an anticipated market launch in Japan in the fourth quarter of 2024.
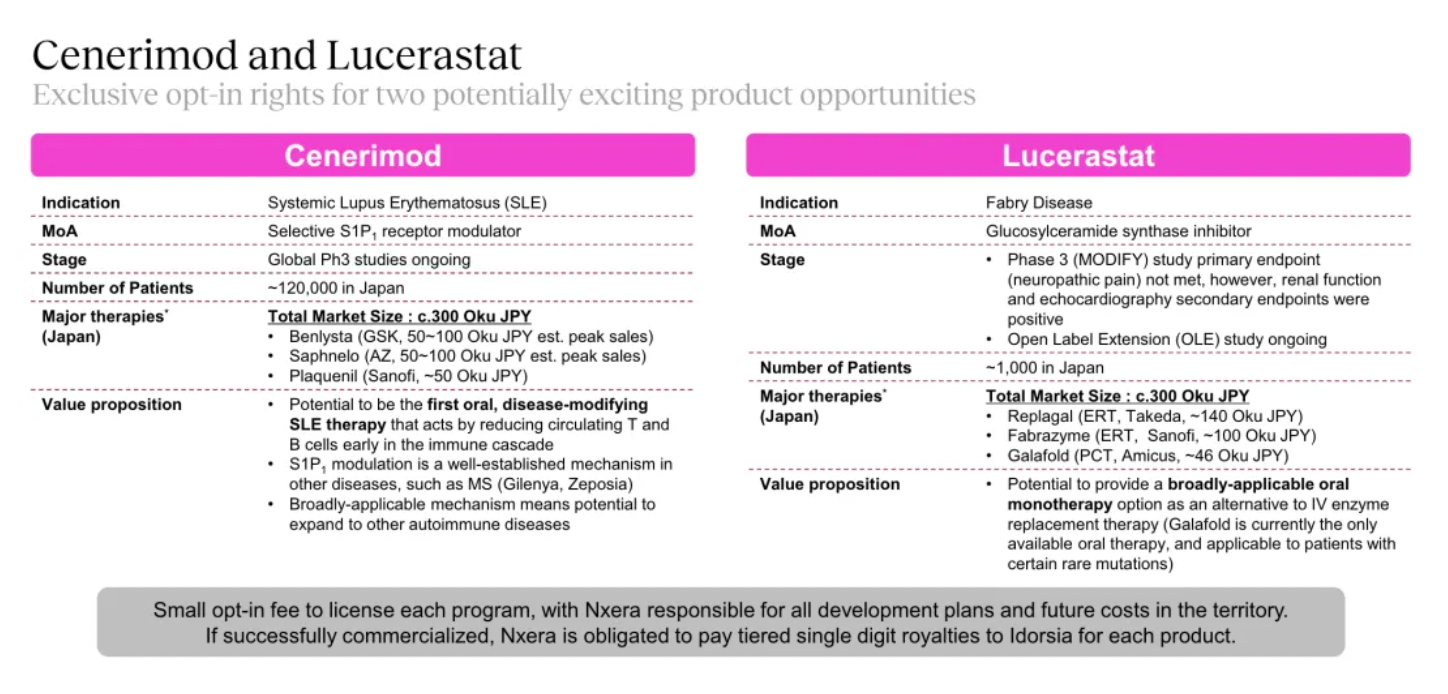
Cenerimod is an S1PR1 receptor modulator currently in Phase III clinical trials for the treatment of Systemic Lupus Erythematosus (SLE).
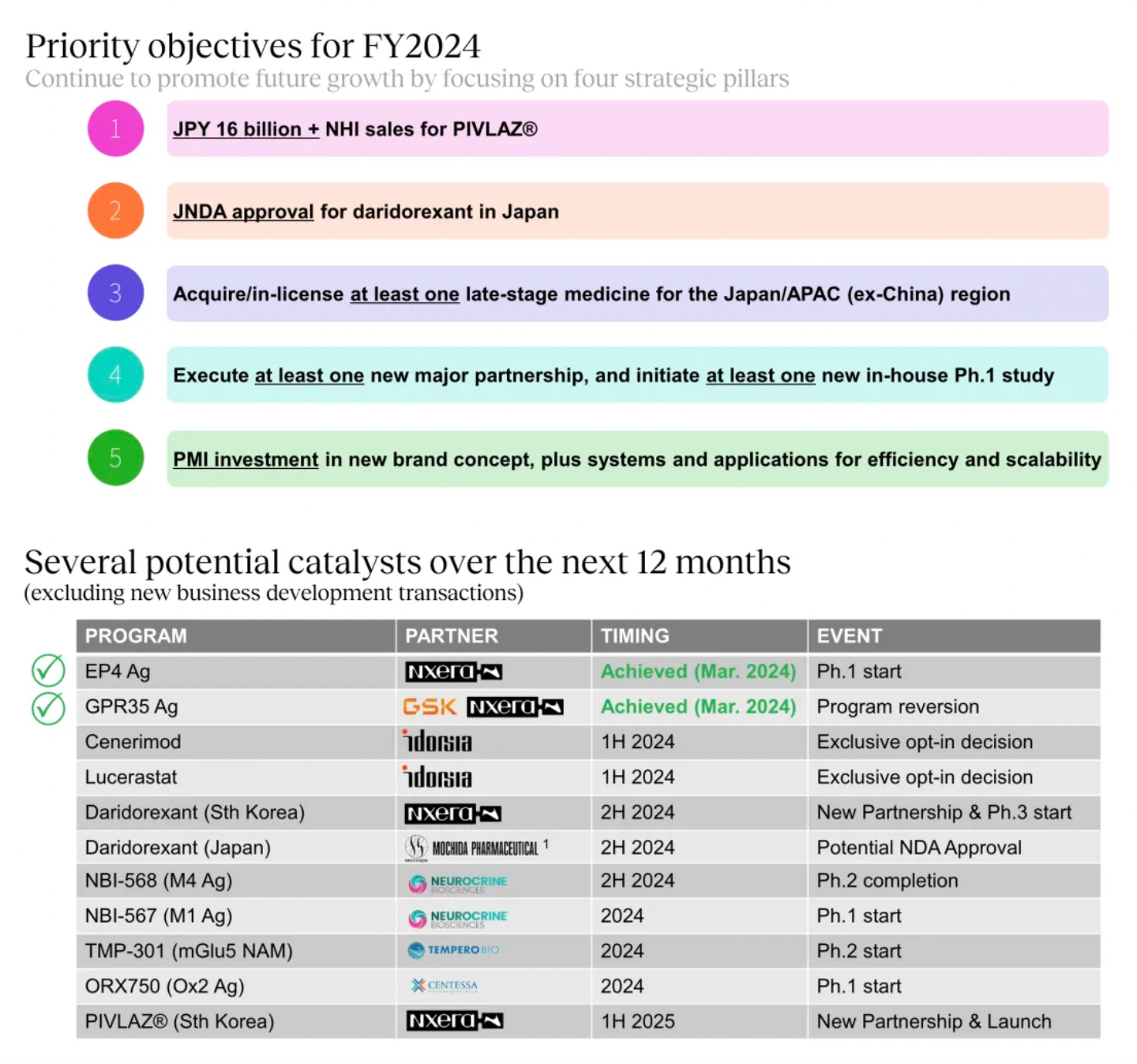
2024 Strategic Objectives
The key strategic goals set for the fiscal year 2024 include executing at least one major partnership, initiating at least one internal Phase 1 study, and acquiring/licensing at least one late-stage drug applicable to Japan and the Asia-Pacific region (excluding China).