Spyre Therapeutics Initiates Phase 1 Trial of SPY001, a Novel Long-acting Anti-α4β7 Antibody for Treating Inflammatory Bowel Disease
Spyre Therapeutics, Inc., a biotechnology firm at the clinical stage that leverages advanced antibody engineering, strategic therapeutic combinations, and precision medicine techniques to achieve enhanced effectiveness and ease in treating Inflammatory Bowel Disease, has announced the commencement of dosing healthy volunteers in its inaugural clinical trial of SPY001, an experimental novel half-life extended anti-α4β7 monoclonal antibody.
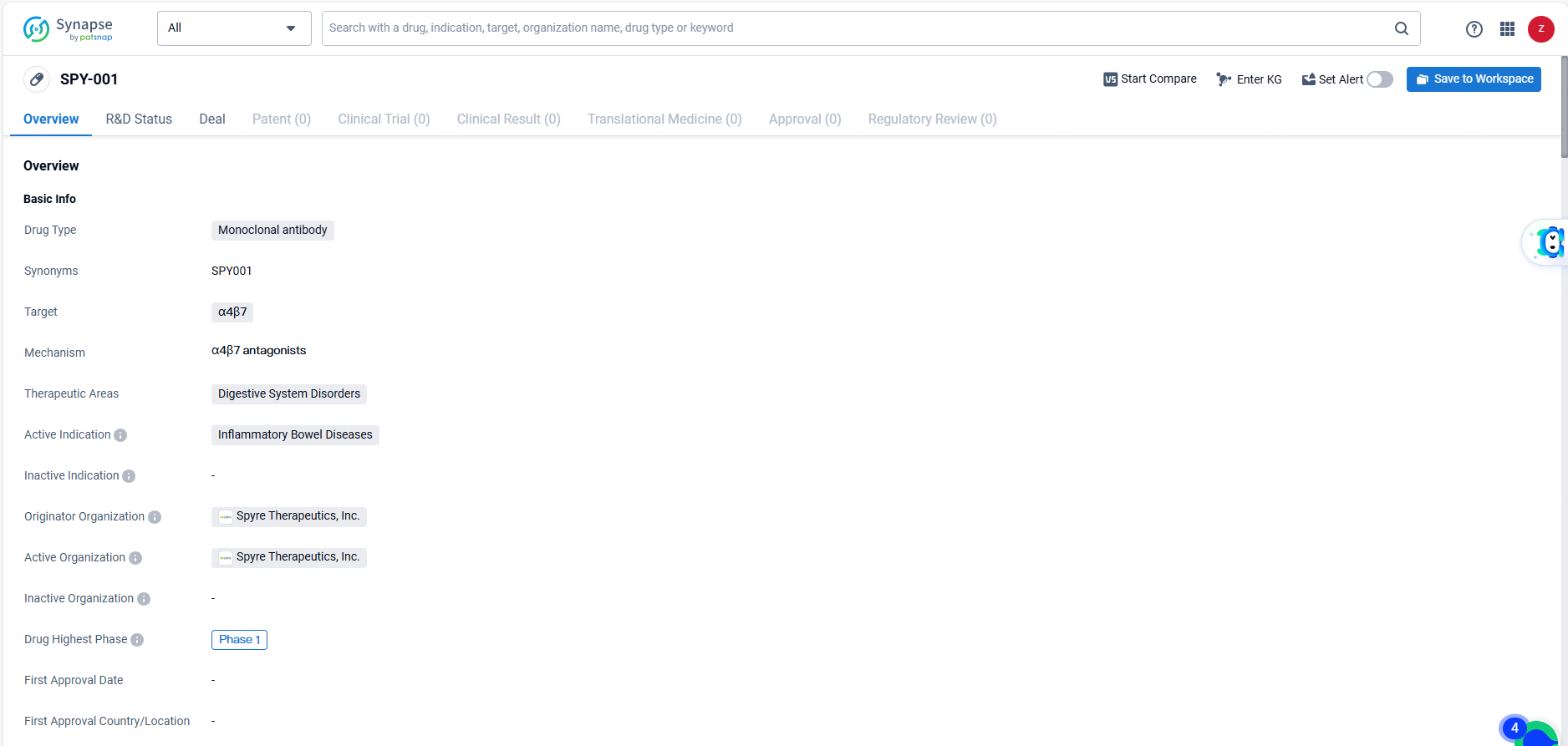
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Within a year of our public launch, the Spyre team has performed with remarkable efficiency, achieving this significant milestone. SPY001 represents the initial program in our trio of impactful mechanisms for IBD—namely α4β7, TL1A, and IL-23—all slated to commence clinical trials in the next twelve months," stated Cameron Turtle, D.Phil., Chief Executive Officer of Spyre.
"We are eager to present interim data for SPY001 by the end of this year, which we believe will demonstrate that SPY001 is well tolerated and has a half-life that supports a convenient Q8-12W subcutaneous dosing regimen, followed by interim results from our TL1A program," Turtle added.
The Phase 1 trial of SPY001 is designed as a double-blind, placebo-controlled study involving healthy volunteers.
It comprises a single-ascending dose (SAD) component and a multi-ascending dose (MAD) component. Approximately 48 healthy adult participants are expected to enroll across four SAD cohorts and two MAD cohorts. The primary aim is to assess safety, with pharmacokinetics (PK) as a secondary measure. We anticipate interim safety and PK data from this trial by the end of 2024. Based on these results, the company aims to move SPY001 to Phase 2 development in 2025.
"Due to its favorable safety profile and gut-selective action, α4β7 inhibition is often the preferred first-line treatment among gastroenterologists, demonstrating superior efficacy compared to TNF inhibition in the VARSITY study," commented Deanna Nguyen, M.D., Senior Vice President of Clinical Development at Spyre. "We are convinced that SPY001's unique safety and efficacy profile, along with a more convenient dosing schedule, could establish it as a crucial component for combination therapies in IBD, targeting other highly active mechanisms like TL1A and IL-23."
SPY001 is an experimental, innovative monoclonal antibody for subcutaneous administration with an extended half-life, aimed at counteracting α4β7 for the potential treatment of IBD. IBD is a persistent inflammatory condition of the gastrointestinal tract, including main disorders such as ulcerative colitis and Crohn’s disease. In the United States, an estimated 2.4 million individuals are currently affected by IBD.
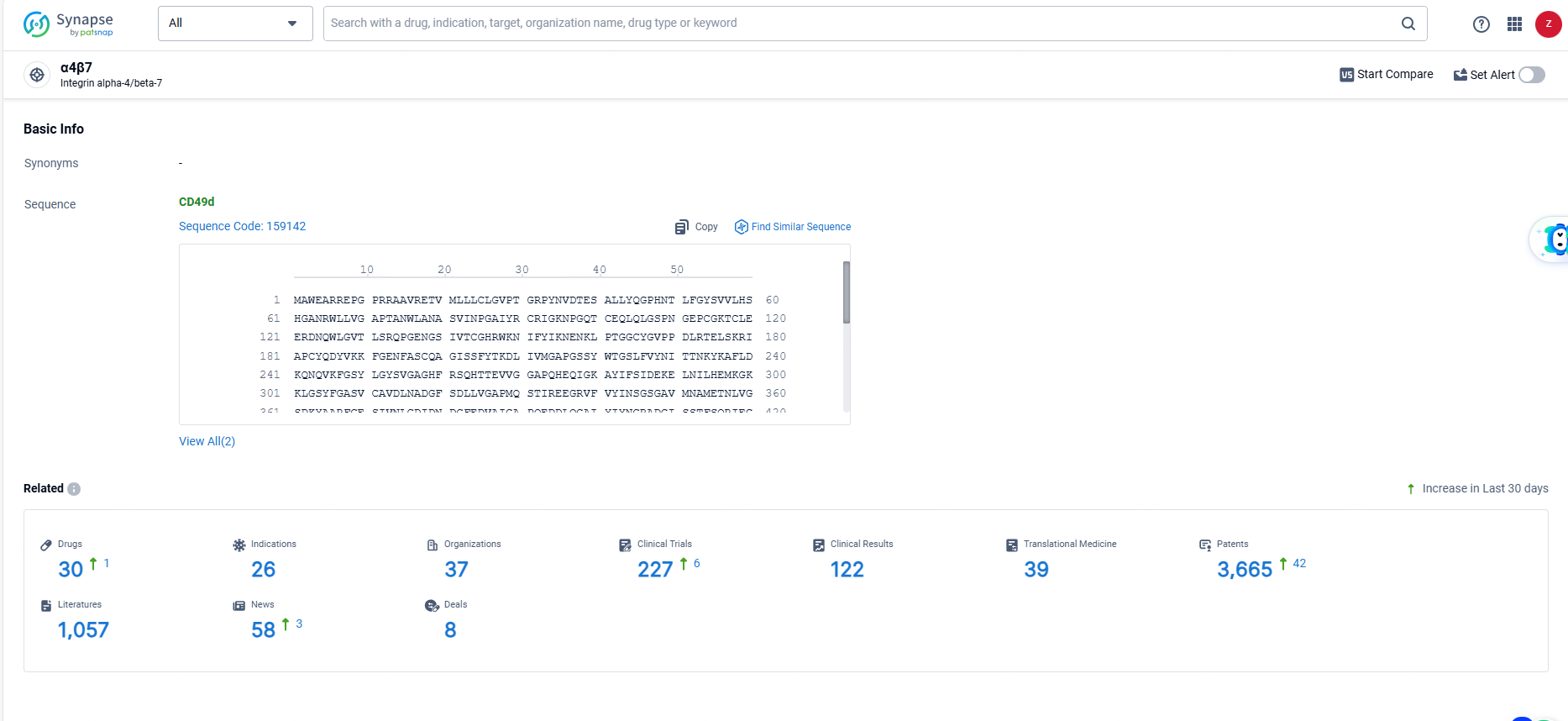
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 23, 2024, there are 30 investigational drugs for the α4β7 target, including 26 indications, 37 R&D institutions involved, with related clinical trials reaching 227, and as many as 3665 patents.
SPY-001 represents a promising advancement in the field of biomedicine, offering potential new treatment options for patients with inflammatory bowel diseases. As the drug progresses through clinical development, further insights into its therapeutic potential and safety profile are expected to emerge, ultimately contributing to the ongoing efforts to address unmet medical needs.