Star Therapeutics Reveals VGA039 Clinical Results for VWD Treatment
Star Therapeutics, a company in the clinical stage of biotechnology focusing on the discovery and development of superior antibodies, has unveiled clinical results from VIVID 1, a Phase 1 trial involving healthy participants. This investigation centers around VGA039, which aims to serve as a universal hemostatic treatment for various bleeding disorders and might become the inaugural subcutaneous therapy for von Willebrand disease.
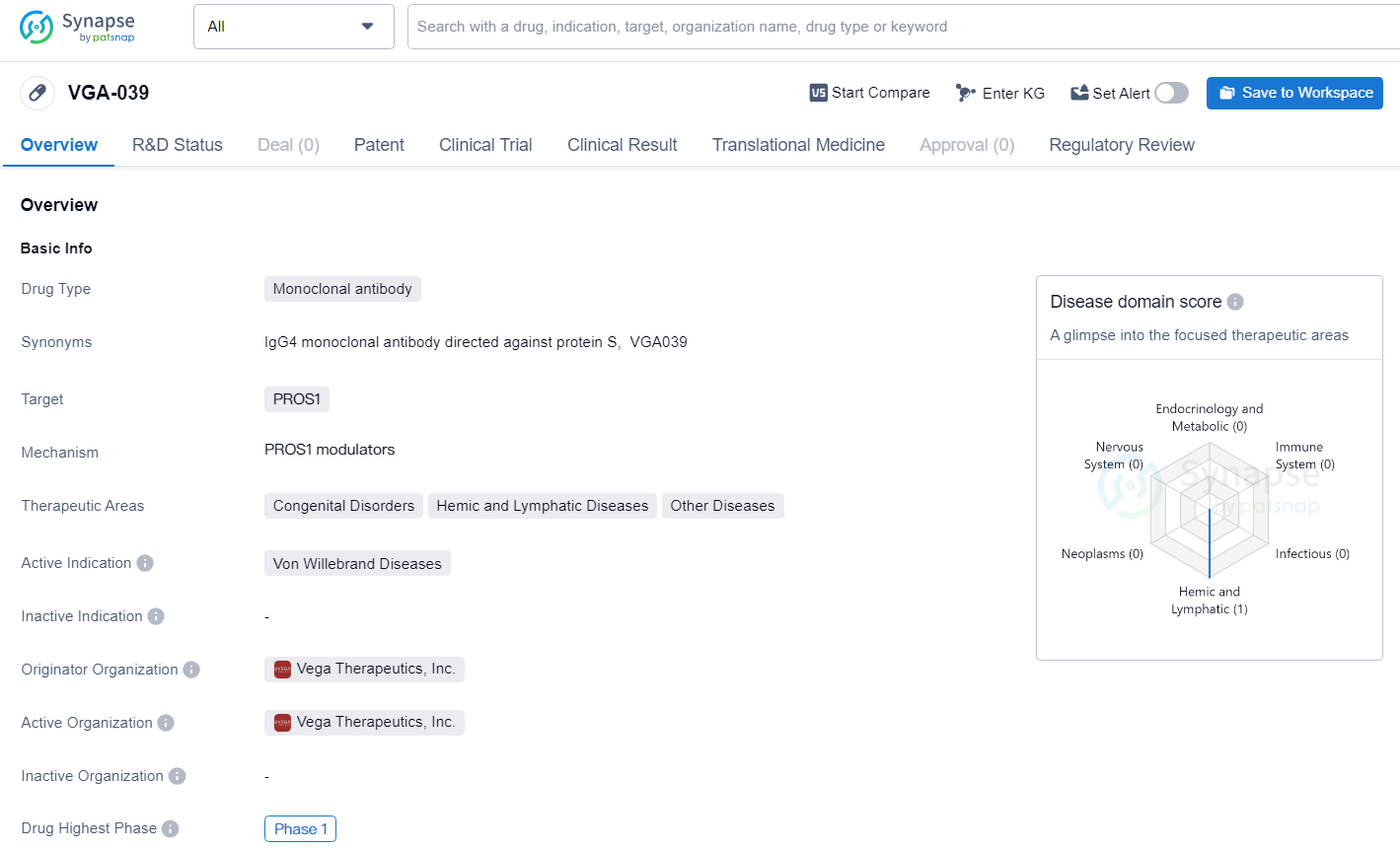
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The clinical data for VGA039 was showcased today in an oral presentation at the 32nd International Society on Thrombosis and Haemostasis Congress, held from June 22-26 in Bangkok, Thailand. The reported data is part of the VIVID clinical program, originating from the initial study phase involving healthy volunteers. The subsequent phase, now in progress, involves VWD patients in the multinational Phase 1 study of VGA039. Results from this Phase 1 study in healthy subjects revealed the pharmacokinetics and pharmacodynamics of VGA039, which support easy subcutaneous administration and demonstrated nearly 100% bioavailability via this route, along with a positive safety and tolerability profile.
"We are enthusiastic about the ongoing development of VGA039 as the prospective first subcutaneous treatment for VWD, aiming to lessen the significant treatment burden affecting VWD patients and their families," stated Jeanette Cesta, Executive Director of the VWD Connect Foundation. "We stand with the patient community in advocating for the progression of new treatments that bring future hope."
"We are thrilled to present these initial clinical results for VGA039. The high subcutaneous bioavailability and favorable pharmacokinetic attributes of VGA039 support its use for convenient subcutaneous prophylactic dosing for VWD," remarked Gary Patou, M.D., Chief Medical Officer at Star Therapeutics. "We are eager to proceed with our VIVID clinical program studying VGA039 in VWD patients."
VGA039 holds promise as a versatile hemostatic therapy potentially addressing various bleeding disorders, including von Willebrand disease. As an antibody therapy administered subcutaneously with convenient dosing, VGA039 could significantly alleviate the treatment burden for patients.
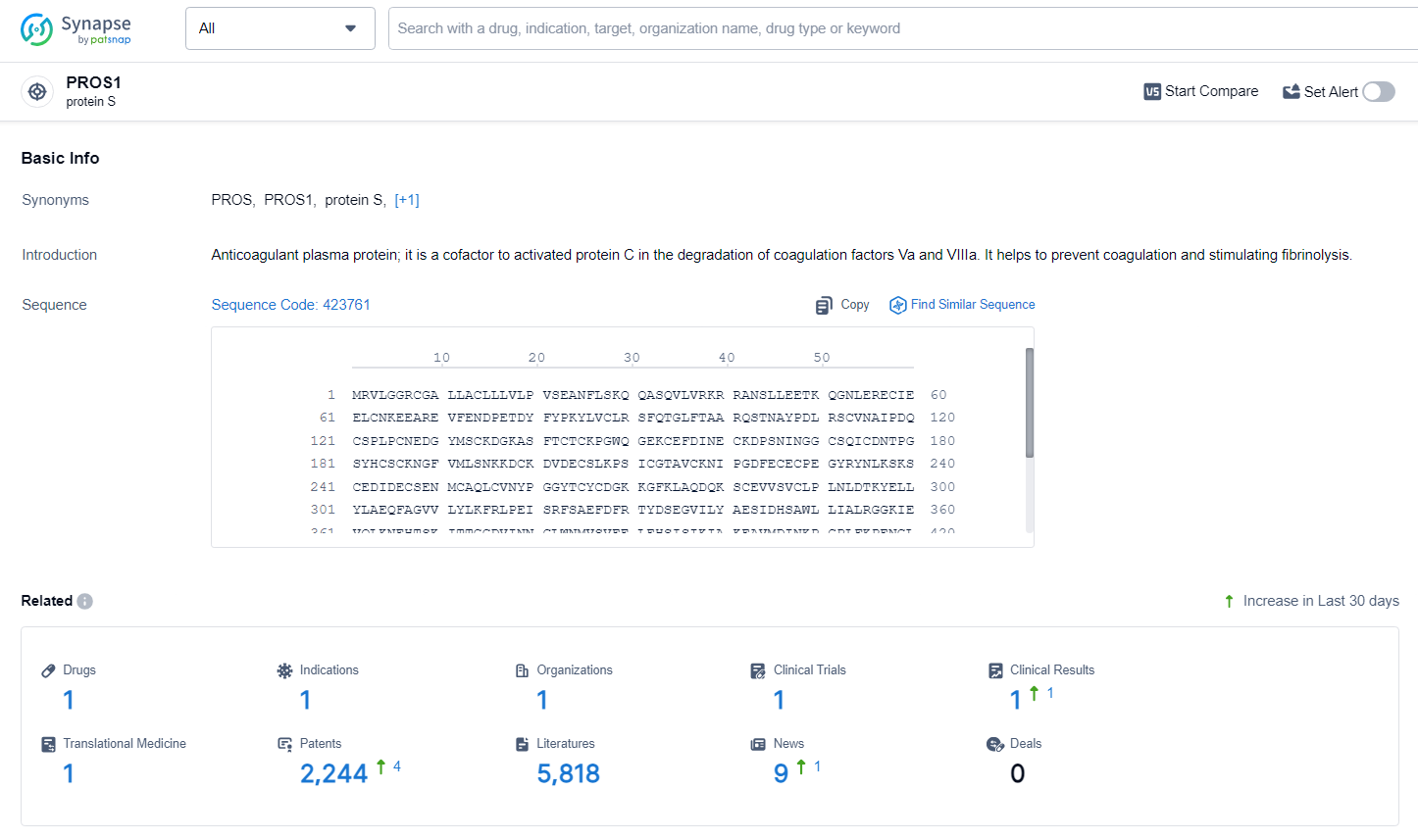
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 1, 2024, there are 1 investigational drugs for the PROS1 target, including 1 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 2244 patents.
VGA-039 is a type of biopharmaceutical drug that is designed to specifically target and bind to PROS1, a protein involved in the regulation of blood clotting. By targeting this protein, VGA-039 aims to address the underlying causes of Von Willebrand Diseases, which are characterized by abnormal bleeding due to a deficiency or dysfunction of the von Willebrand factor.VGA-039 holds promise as a potential therapeutic option for individuals affected by Von Willebrand Diseases and related disorders, and its progression through clinical development will be of interest to stakeholders in the pharmaceutical and biomedicine industries.