Early Phase 1 Results of WTX-330 on Advanced Solid Tumors and Non-Hodgkin Lymphoma Presented by Werewolf Therapeutics
Werewolf Therapeutics, Inc., a forward-thinking biopharmaceutical firm developing conditionally activated therapies designed to enhance the body's immune response against cancer, revealed preliminary data from the Phase 1 clinical study of WTX-330. This conditionally activated IL-12 INDUKINE™ molecule is being tested as a standalone treatment for patients with locally advanced or metastatic solid tumors or non-Hodgkin lymphoma that are unresponsive or resistant to immunotherapy.
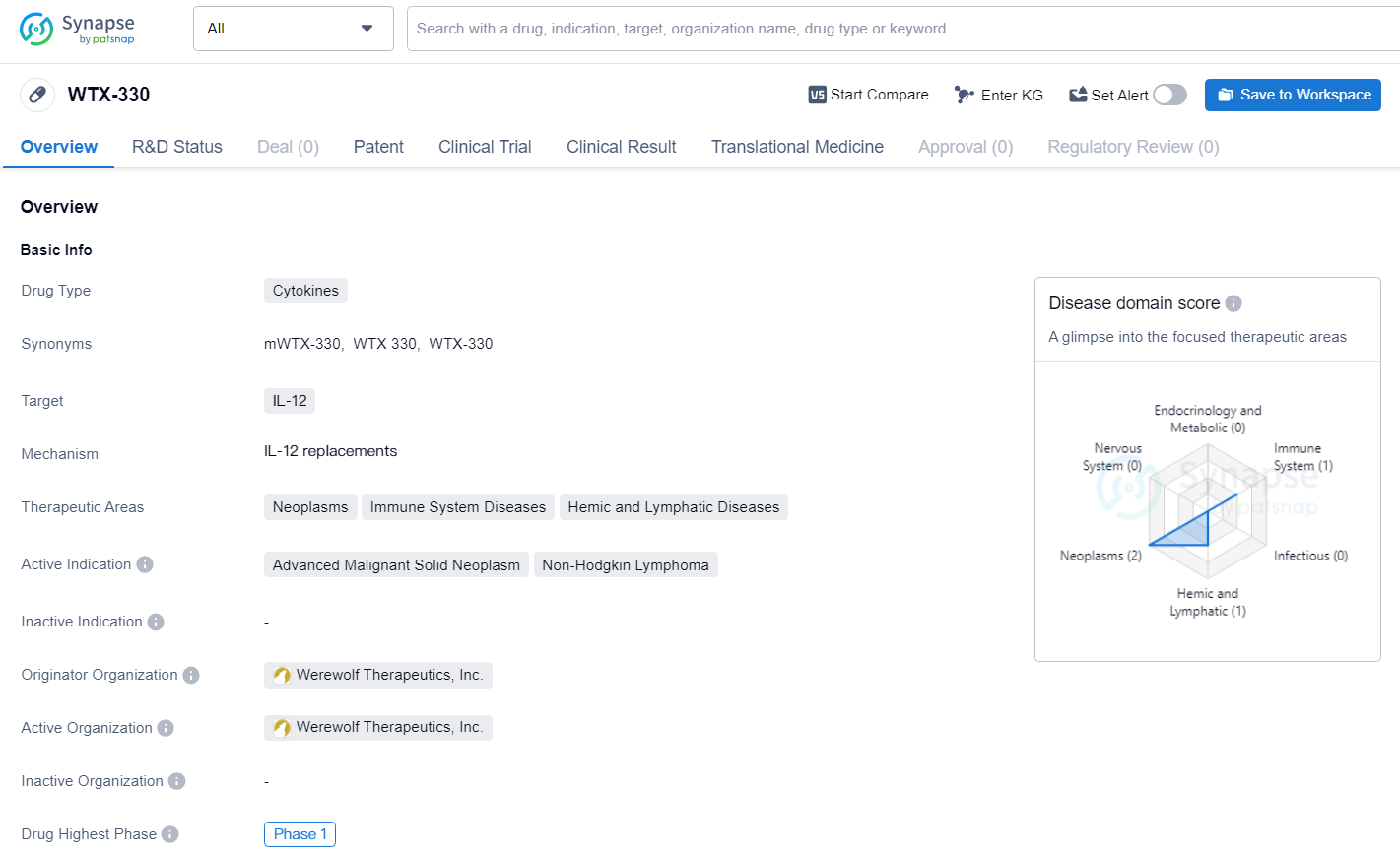
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“Our primary objective at Werewolf involves pushing forward the development of next-generation, transformative immuno-stimulatory therapies,” remarked Daniel J. Hicklin, Ph.D., President and CEO of Werewolf. “The initial clinical results indicate that WTX-330 exhibits encouraging tolerability and efficacy signals in patients with advanced, heavily pretreated solid tumors. We are eager to continue the progress of WTX-330 and explore its potential clinical benefits.”
IL-12 therapy represents a promising approach for cancers resistant to the immune system, but its use has historically been hampered by high toxicity, similar to many cytokines. Werewolf is working on an innovative, conditionally activated IL-12 therapy, WTX-330, which aims to overcome this challenge using its systemically delivered, tissue-specific targeting technology. This method enhances the therapeutic index, allowing effective doses to be administered and achieve clinical effects.
“This appears to be the first instance where a full-strength, systemically administered IL-12 molecule has shown clinical benefits at therapeutically meaningful doses with reduced severe toxicity in an outpatient context,” commented Randi Isaacs, M.D., Chief Medical Officer of Werewolf. “We are heartened by these preliminary outcomes and plan to share more insights on safety, biomarkers, and antitumor activity from patients in expansion cohorts at a medical conference in the last quarter of 2024.”
Interleukin-12 is widely recognized as a potential antitumor therapeutic due to its capabilities, including the activation of natural killer cells, NK T cells, and CD8+ T cells, enhancement of dendritic cell antigen presentation, and production of IFN-γ.
WTX-330 is designed as a systemically dosed prodrug capable of delivering fully active IL-12 selectively into the tumor microenvironment through targeted intratumoral activation of the INDUKINE molecule. This approach could expand the therapeutic window and enhance local immune response against the tumor.
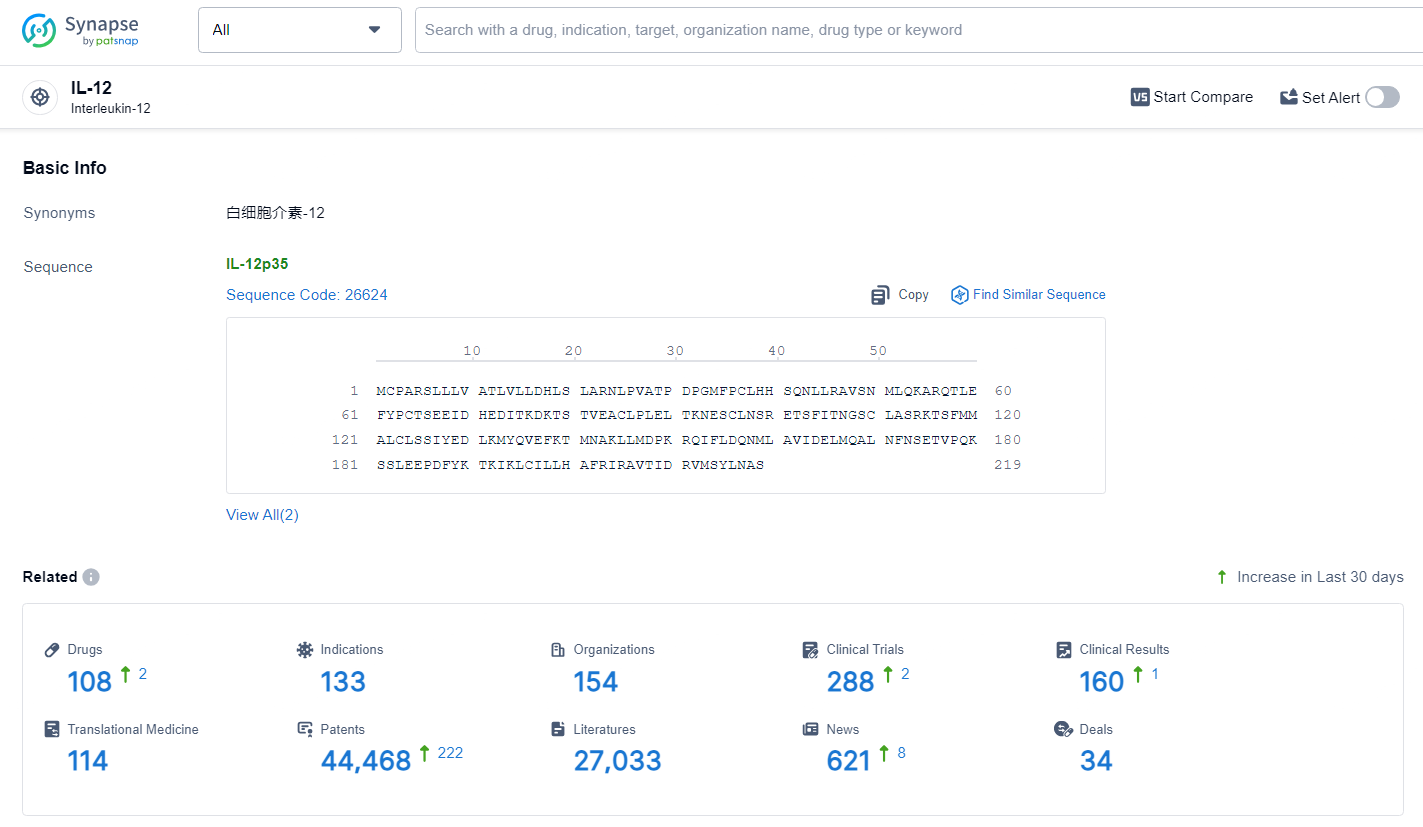
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 1, 2024, there are 108 investigational drugs for the IL-12 target, including 133 indications, 154 R&D institutions involved, with related clinical trials reaching 288, and as many as 44468 patents.
WTX-330 is a cytokine drug targeting IL-12, developed by Werewolf Therapeutics, Inc. for the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases, with a specific focus on advanced malignant solid neoplasm and non-Hodgkin lymphoma. The drug is currently in Phase 1 of clinical development, representing an early stage in its potential journey to becoming a therapeutic option for patients with these challenging conditions.