Strontium ranelate: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Strontium ranelate's R&D Progress
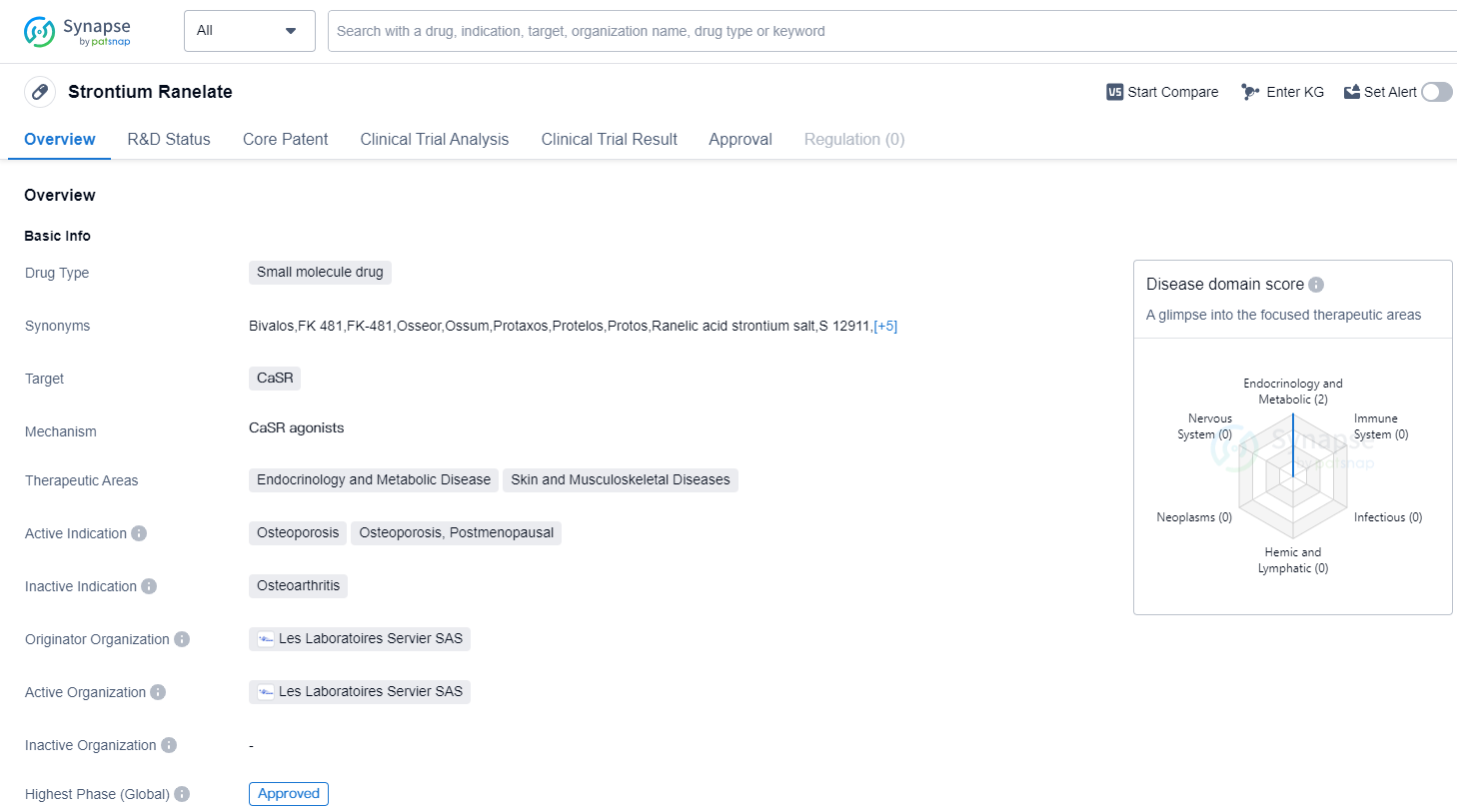
Strontium Ranelate is a small molecule drug that targets the Calcium Sensing Receptor (CaSR). It is primarily used in the treatment of osteoporosis, specifically in postmenopausal women. The highest R&D phase of this drug is approved.
The therapeutic areas for Strontium Ranelate include endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. This indicates that the drug may have potential applications beyond osteoporosis, although further research and clinical trials may be required to explore these possibilities.
The drug was first approved in the European Union in September 2004, making it available to patients in that region. It is important to note that the information provided does not specify whether the drug has received approval in other countries or regions.
Strontium Ranelate was developed by Les Laboratoires Servier SAS, an originator organization in the pharmaceutical industry. As an approved drug, it has undergone rigorous testing and evaluation to ensure its safety and efficacy for patients.
The fact that Strontium Ranelate has reached the highest phase of development which is approved globally. This suggests that the drug has demonstrated positive results in clinical trials and has met the necessary regulatory requirements for approval.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for strontium ranelate: CaSR agonists
CaSR agonists are a type of drug that activate the Calcium-Sensing Receptor (CaSR). The CaSR is a G-protein coupled receptor found on the surface of various cells in the body, including cells in the parathyroid gland, kidneys, and intestines. When CaSR is activated by an agonist, it triggers a signaling cascade within the cell, leading to various physiological responses.
In the context of biomedicine, CaSR agonists are primarily used in the treatment of disorders related to calcium metabolism, such as primary and secondary hyperparathyroidism. By activating the CaSR, these agonists help regulate the levels of calcium in the body, which is essential for maintaining bone health, nerve function, and muscle contraction.
CaSR agonists can also have potential applications in other areas of medicine, such as the treatment of certain types of cancer. Research is ongoing to explore their therapeutic potential in conditions like colon cancer and breast cancer.
Overall, CaSR agonists play a crucial role in modulating calcium homeostasis and have therapeutic implications in various biomedical conditions.
Drug Target R&D Trends for strontium ranelate
The Calcium Sensing Receptor (CaSR) is a protein found in various tissues of the human body, including the parathyroid glands, kidneys, and gastrointestinal tract. It plays a crucial role in maintaining calcium homeostasis by sensing changes in extracellular calcium levels. When calcium levels are low, CaSR stimulates the release of parathyroid hormone (PTH) to increase calcium absorption from the bones and kidneys. Conversely, when calcium levels are high, CaSR inhibits PTH release and promotes calcium excretion. Additionally, CaSR is involved in regulating other physiological processes, such as cell proliferation, differentiation, and hormone secretion. Understanding the role of CaSR is essential for developing therapies targeting calcium-related disorders.
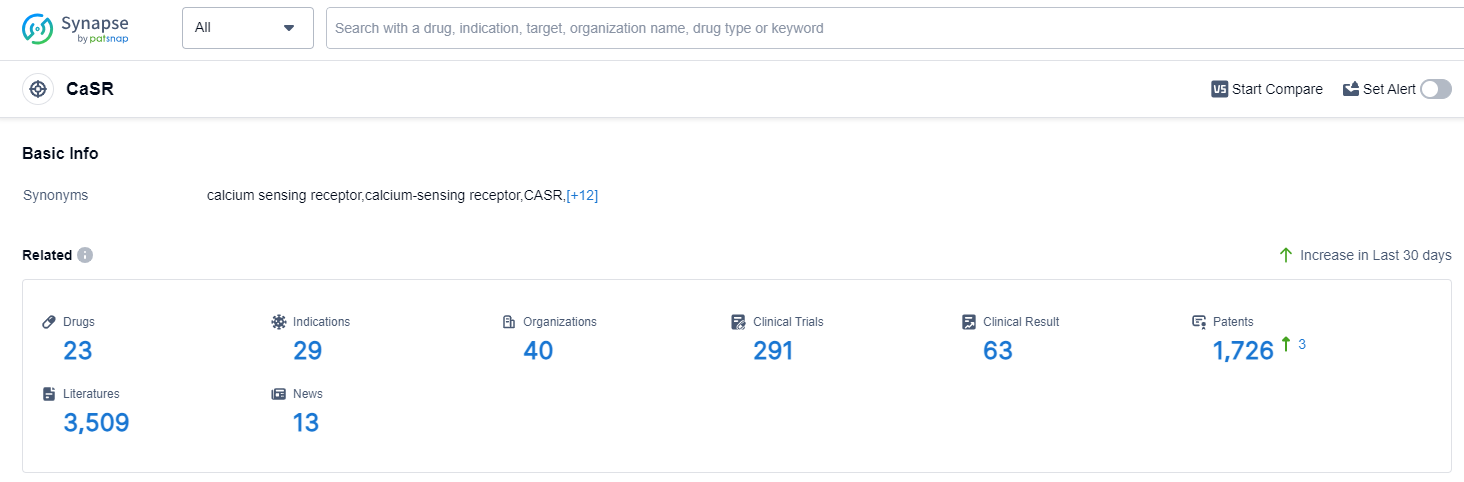
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 23 CaSR drugs worldwide, from 40 organizations, covering 29 indications, and conducting 291 clinical trials.
The analysis of the current competitive landscape of target CaSR reveals that Amgen, Inc., Kirin Holdings Co., Ltd., and Ascendis Pharma A/S are the companies growing fastest under this target. The highest stage of development is seen in Amgen, Inc., with multiple drugs approved and in advanced stages of development. The approved indications for drugs targeting CaSR include various conditions related to parathyroid disorders, osteoporosis, and kidney failure. Small molecule drugs are progressing rapidly, indicating intense competition in the market. Japan, European Union, China, and Russia are the countries/locations with significant progress in the development of drugs targeting CaSR. China, in particular, has shown progress in the development of drugs for this target. Overall, the target CaSR presents a competitive landscape with multiple companies and drug types involved in the research and development of drugs for various indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Strontium Ranelate is a small molecule drug that targets the CaSR and is primarily used in the treatment of osteoporosis. It has been approved for use in the European Union and China, with its first approval dating back to September 2004. The drug was developed by Les Laboratoires Servier SAS and has shown promise in the therapeutic areas of endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. Further research and exploration may be needed to fully understand the drug's potential beyond its current approved indications.