Structure Therapeutics GLP-1R Small Molecule Agonist GSBR-1290 Preclinical and Clinical Data
On June 3, the official website of Structure Therapeutics announced top-line data from its 12-week clinical Phase 2a study of the investigational drug GSBR-1290 in obesity, along with positive top-line results from a pharmacokinetic (PK) study comparing the capsule-to-tablet formulation transition.

In the Phase 2a obesity study, GSBR-1290 demonstrated clinically meaningful and statistically significant weight loss. At week 12, participants on GSBR-1290 showed a mean weight reduction of 6.2% compared to placebo (p<0.0001). Additionally, 67% of participants receiving GSBR-1290 achieved at least a 6% weight reduction, and 33% achieved at least a 10% weight reduction by week 12, while no participants in the placebo group achieved these benchmarks.
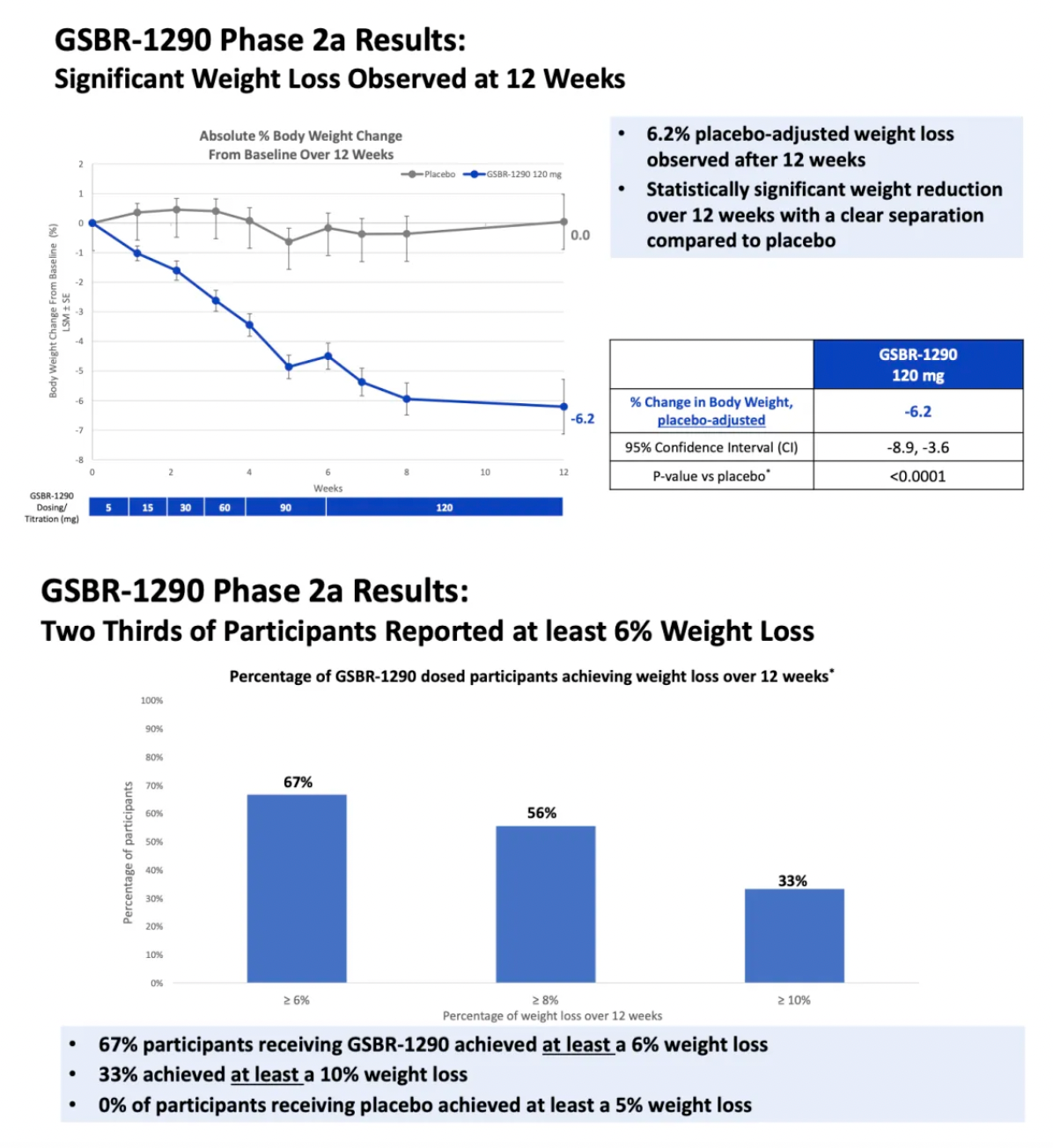
The company's capsule-to-tablet PK study aimed at exploring the new tablet formulation of GSBR-1290 indicated an average weight loss of up to 6.9% compared to placebo at week 12 (p<0.0001).
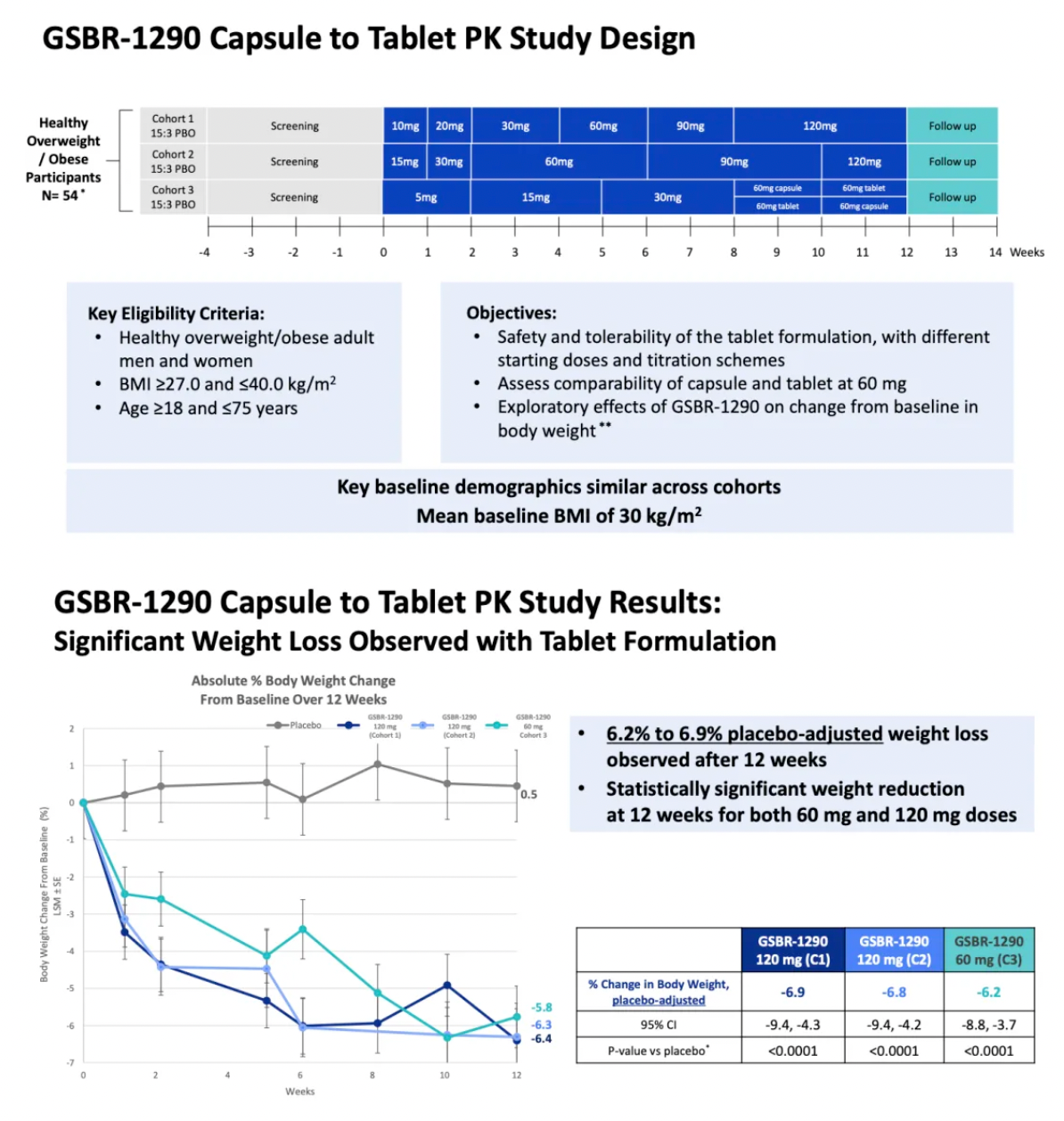
The tablet formulation exhibited comparable exposure levels to the previous capsule formulation, with pharmacokinetic data indicating that GSBR-1290 has dose-proportional exposure and supports once-daily dosing.
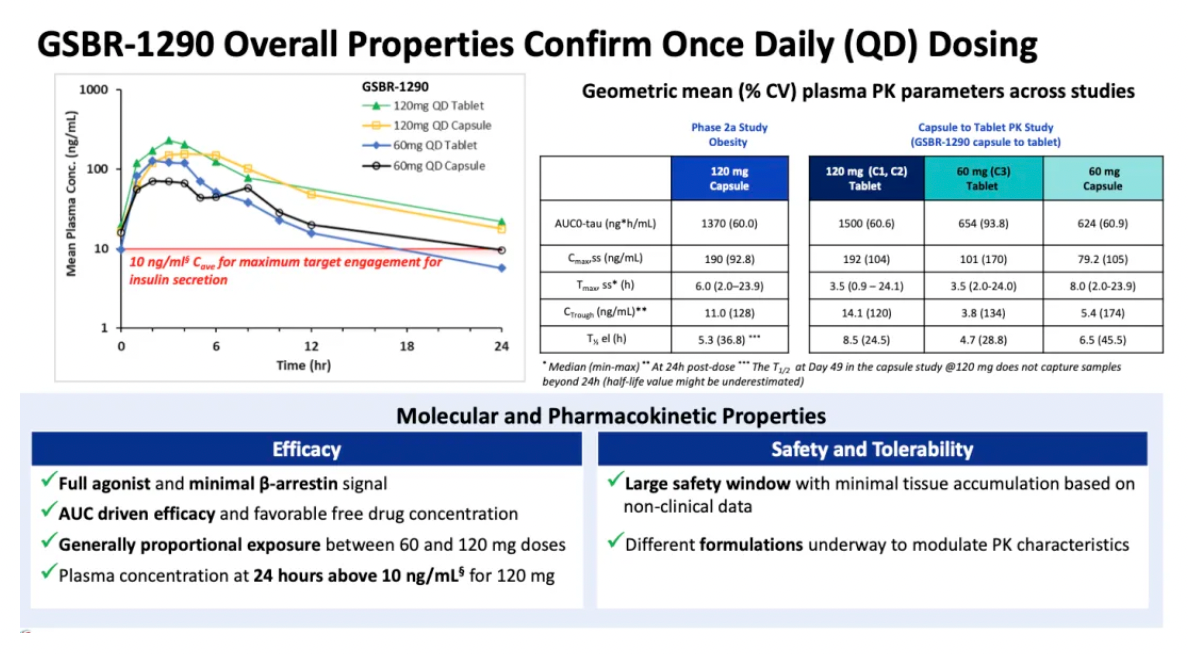
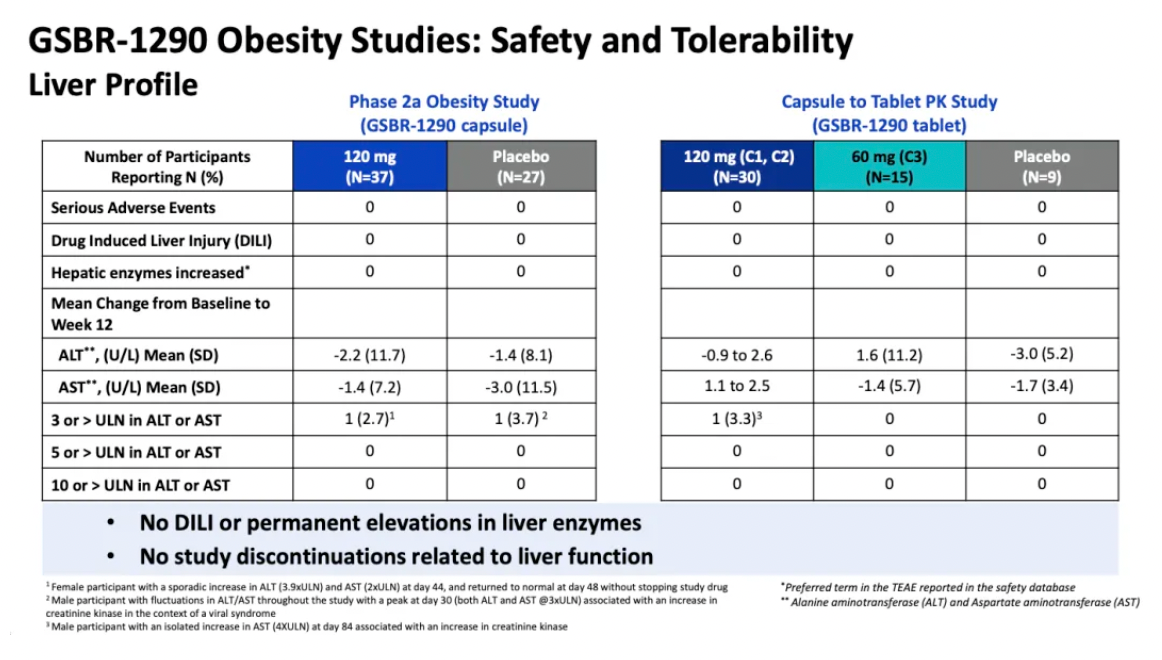
The repeated daily administration of GSBR-1290 at doses up to 120 mg exhibited an overall good safety and tolerability profile. As a GLP-1 receptor agonist, the most common adverse events (AEs) were related to the gastrointestinal (GI) system, with nausea and vomiting being the most frequently reported AEs. Gastrointestinal-related adverse events typically emerged early in the treatment period and generally diminished upon dose adjustment. Study discontinuations due to adverse events accounted for 5% in the Phase IIa obesity study and 11% in the capsule-to-tablet PK study. No cases of drug-induced liver injury or sustained elevation in liver enzymes were reported in either study.
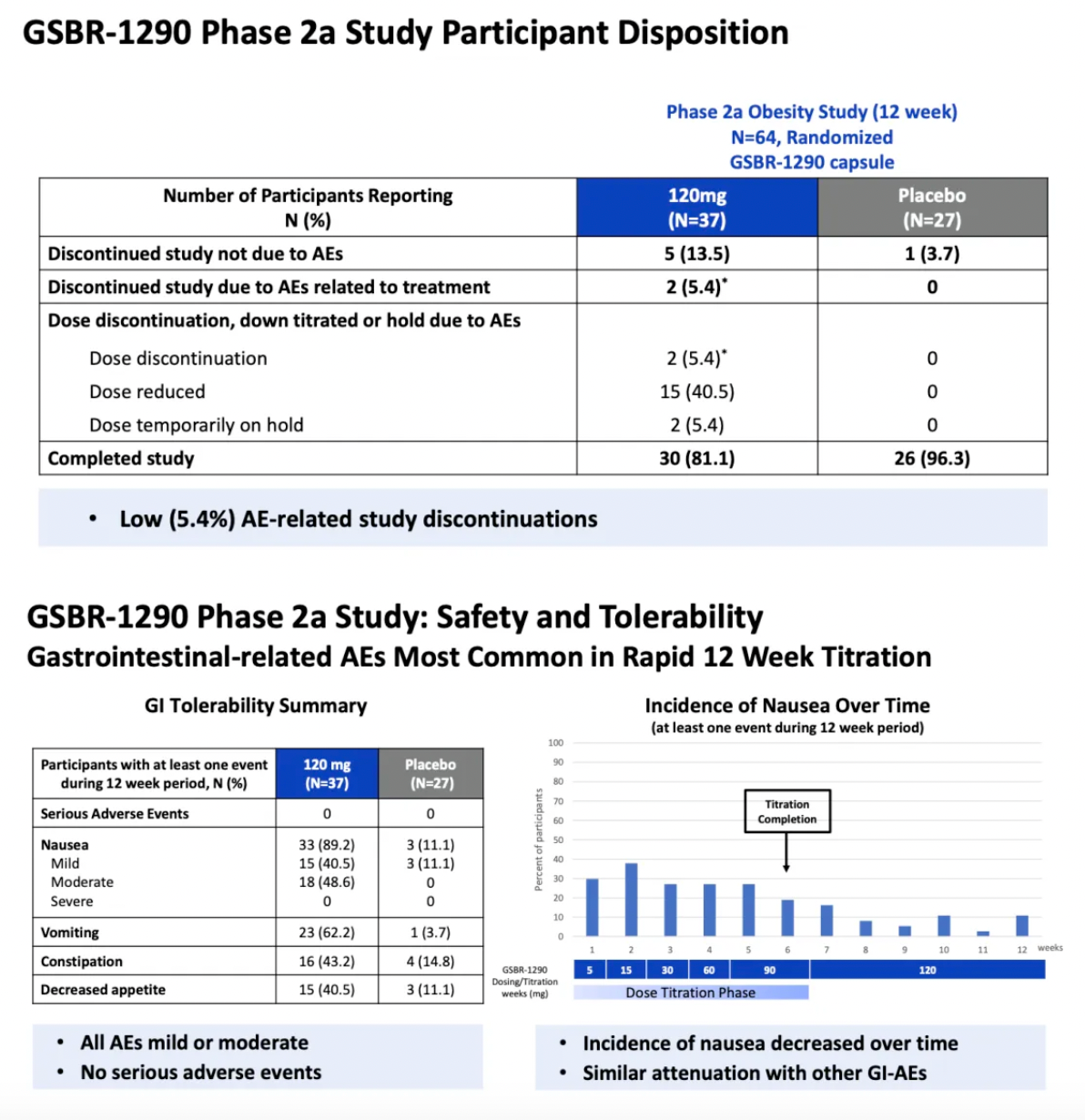
GSBR-1290 Phase 2a Proof-of-Concept Clinical Trial
In November 2023, the official website of Structure Therapeutics announced a comprehensive update on the GSBR-1290 project data, including proof-of-concept data from the Phase 2a clinical study.

According to the materials published on the official website, GSBR-1290 is undergoing a 12-week randomized, double-blind, placebo-controlled Phase 2a clinical trial to evaluate the therapeutic effect of the drug in patients with type 2 diabetes mellitus (T2DM) and obesity. A total of 94 participants have been recruited, with 54 participants in the T2DM group and an initial recruitment of 40 participants in the obesity cohort.
Regarding safety, most reported adverse events (AEs) (88% to 96%, depending on the study arm) were mild to moderate in severity. Among the 60 participants treated with GSBR-1290, only one participant discontinued the study due to an AE related to the study drug (0 in the obesity group, 1 in the T2DM group, representing 2.8%).
In the T2DM cohort, GSBR-1290 demonstrated a statistically significant reduction in HbA1c at week 12 (a decrease of -1.01% to -1.02%, placebo-adjusted). At week 12, there was also a statistically and clinically significant reduction in body weight (a decrease of -3.26% to -3.51%, placebo-adjusted). In the obesity cohort, there was a significant statistical and clinical reduction in body weight at week 8 (-4.74%, placebo-adjusted). Throughout the 8-week treatment period, there was a continuous decrease in body weight.
Following the release of the related clinical data, Structure Therapeutics' stock price plummeted, nearly halving. After Pfizer's setback, Structure Therapeutics' clinical development data fell far below industry expectations, sparking widespread media discussion.
It had been less than six months since Structure Therapeutics disclosed preclinical data at the ADA conference in June. The industry landscape has changed dramatically, with Pfizer's two core GLP-1R small molecule agonists, Danuglipron and Lotiglipron, both failing, while Structure Therapeutics' stock experienced a rollercoaster-like fluctuation, instilling a sense of panic among investors.
Clinical Phase 1b Results of GSBR-1290
Based on the information provided on the Structure Therapeutics website, participants in the clinical phase 1b multiple-dose study were randomized in a 3:1 ratio into three dosage groups of GSBR-1290 with target doses of 30 mg, 60 mg, or 90 mg, and a placebo group. The treatment was administered orally on a daily basis over a period of 28 days. Compared to baseline, the average weight reduction with GSBR-1290 was up to 4.9 kg, with a placebo-adjusted weight reduction of up to 4.9%.
Regarding safety, no severe side effects were observed across multiple dosage groups.
No participants discontinued the medication due to adverse reactions. The majority of reported adverse events were mild, and no serious adverse events were identified. As expected, the primary adverse events were related to the gastrointestinal system, with nausea and vomiting being the most common. These events were observed more frequently in the 60 mg and 90 mg dosage groups compared to the placebo group. There were no clinically significant changes in liver function tests.
Drug Discovery and Preclinical Research
GSBR-1290 is an orally available biased small molecule GLP-1 receptor agonist, initially intended for the treatment of Type 2 Diabetes Mellitus (T2DM) and obesity. Given its significant preclinical activity and oral bioavailability, GSBR-1290 has the potential to become a differentiated treatment option without the need for dietary restrictions or concurrent therapeutic regimens.
GSBR-1290 was designed using an in-house structure-based drug discovery platform. Multiple small molecules that interact with the GLP-1 receptor structure have been generated to guide iterative chemical design efforts. GSBR-1290 is also designed as a biased GPCR agonist that activates the G protein pathway at therapeutic doses without eliciting β-Arrestin signaling, thereby avoiding receptor internalization and desensitization.
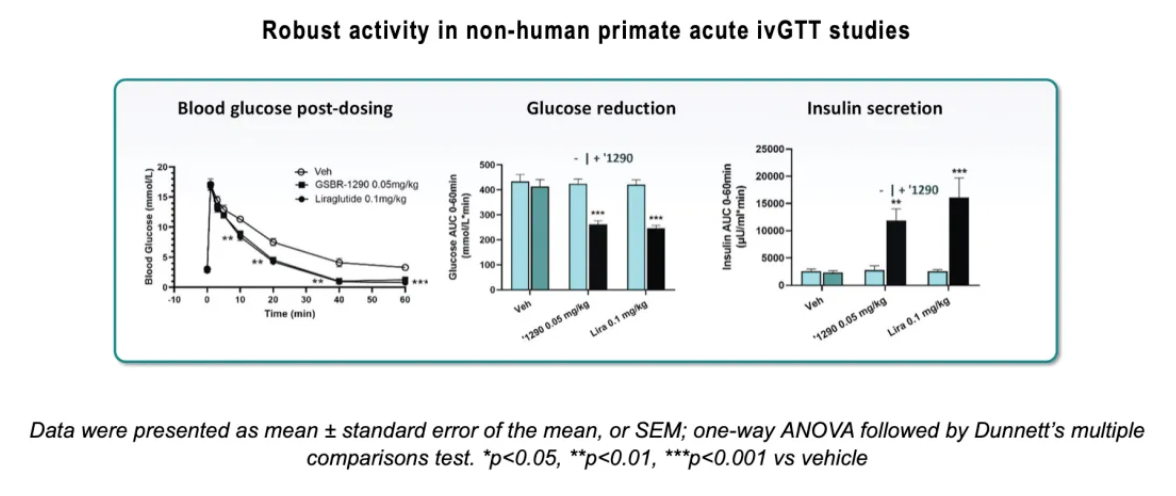
In intravenous glucose tolerance tests (ivGTT) in non-human primates (NHPs), GSBR-1290 increased glucose-dependent insulin secretion to levels comparable with those of the approved injectable GLP-1 receptor agonist Liraglutide. In a repeated food intake study in NHPs, GSBR-1290 showed a significant weight loss effect compared to placebo, surpassing the effects observed with Liraglutide.
In ivGTTs in NHPs, intravenous administration of GSBR-1290 (0.05 mg/kg) or Liraglutide (0.1 mg/kg) was followed by glucose infusion five minutes later. Plasma samples were collected at indicated time points to assess insulin and glucose levels. GSBR-1290 showed statistically significant reductions in blood glucose concentrations by stimulating insulin secretion in a glucose-dependent manner, similar to the effects of Liraglutide, when administered at doses equivalent to approved human doses.
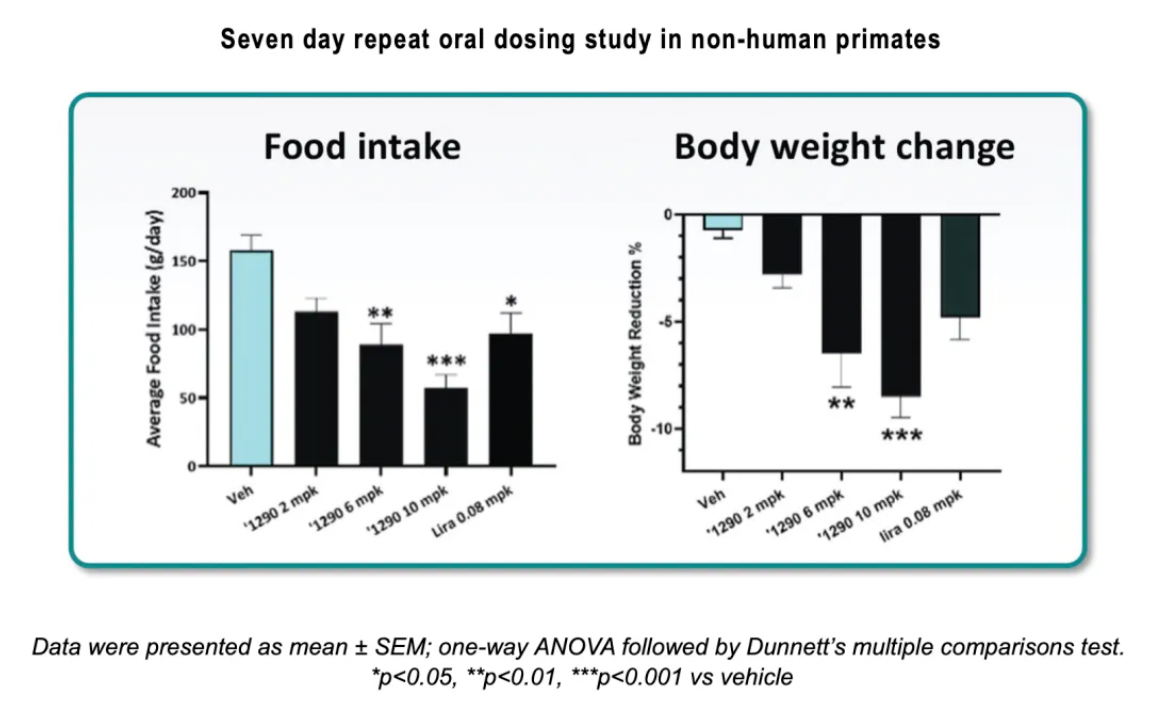
In a seven-day oral repeat-dose study in non-human primates (NHP), the oral administration of GSBR-1290 was evaluated at daily doses of 2 mg/kg, 6 mg/kg, and 10 mg/kg, in comparison with a placebo and Liraglutide. At all doses, GSBR-1290 demonstrated a statistically significant reduction in blood glucose levels compared to the placebo, and it was comparable to Liraglutide. Similarly, all doses significantly increased insulin secretion. At doses of 6 mg/kg and 10 mg/kg, there was a statistically significant reduction in average food intake over the first six days of the study relative to the placebo. At the 10 mg/kg dose of GSBR-1290, the average food intake from day one to day six was only 59% of that in the Liraglutide group. Furthermore, GSBR-1290 at doses of 6 mg/kg and 10 mg/kg showed significant weight loss relative to the placebo and exceeded that of Liraglutide; the highest dose of GSBR-1290 led to an average weight reduction of more than 8% from baseline within one week.
Based on a 28-day GLP toxicology study, GSBR-1290 was shown to have overall good tolerability, with a No Observable Adverse Effect Level (NOAEL) of 1000 mg/kg/day in rats. According to the 28-day GLP toxicology study in rats, the estimated therapeutic window exceeds 1000-fold.
The researchers also conducted a preclinical comparative study of GSBR-1290 and PF-06882961, the latter being a clinical-stage compound under development by Pfizer Inc.




