EU Approves Bristol Myers Squibb's Opdivo® Combo for Advanced Urothelial Carcinoma
Bristol Myers Squibb shared that the European Commission has given the green light to Opdivo® (nivolumab) together with cisplatin and gemcitabine for the initial treatment of adult patients facing unresectable or metastatic urothelial carcinoma.
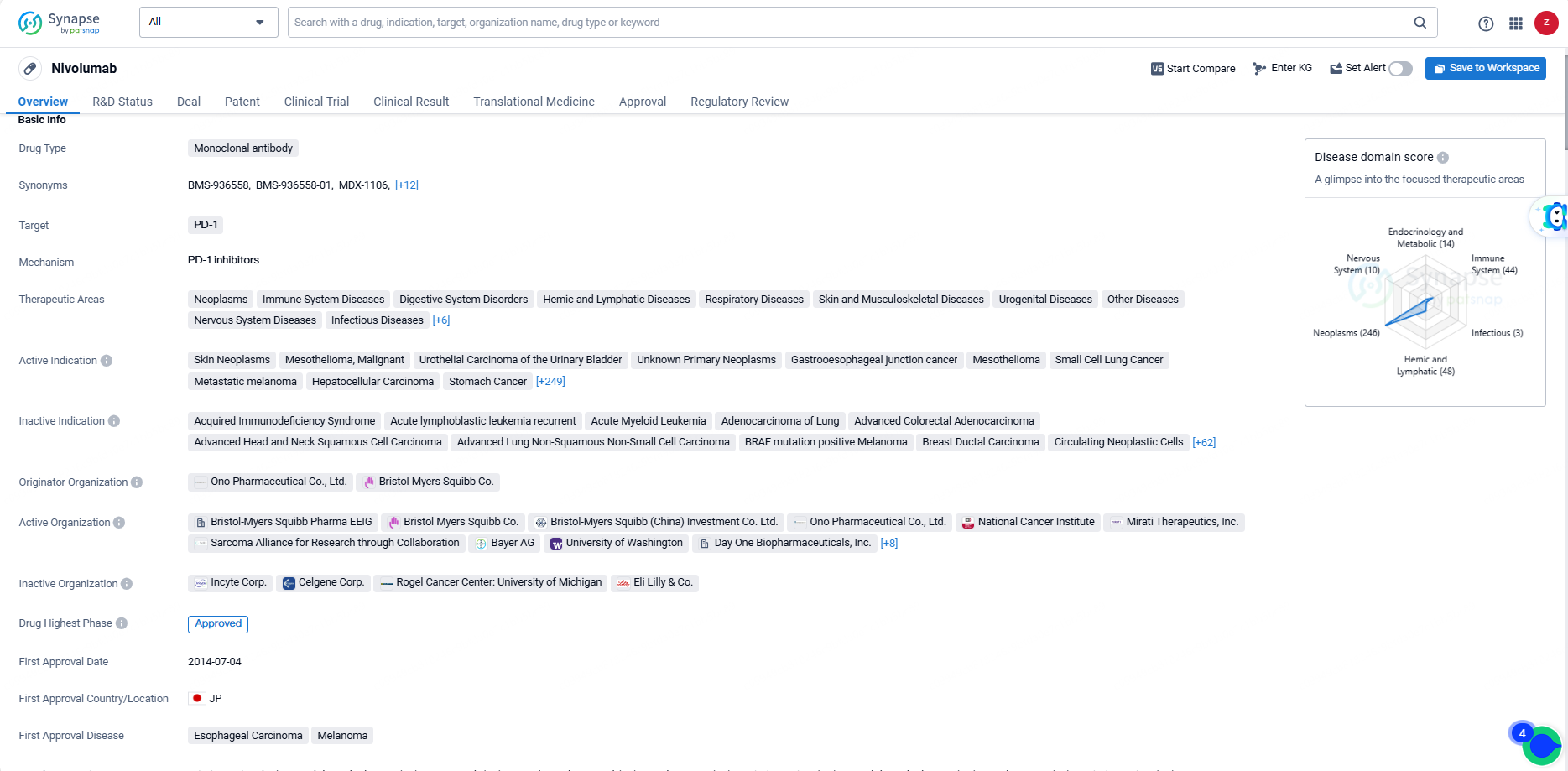
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
With this endorsement, the combination of Opdivo, cisplatin, and gemcitabine has become the first jointly approved immunotherapy and chemotherapy for the initial treatment of adult patients with unresectable or metastatic UC within the European Union.
“Following today's endorsement by the EC, we're delighted to offer Opdivo with chemotherapy to eligible individuals facing unresectable or metastatic UC,” stated Dana Walker, M.D., M.S.C.E., vice president and global program lead in gastrointestinal and genitourinary cancers at Bristol Myers Squibb. “This milestone represents significant progress for these patients and underscores our commitment to developing and delivering new treatment options for challenging cancers. We deeply appreciate the efforts of the patients, their families, and the researchers and staff involved in this crucial study.”
The EC's decision is founded on data from the CheckMate -901 trial, which analyzed the use of Opdivo with cisplatin and gemcitabine and was showcased at the European Society of Medical Oncology Congress 2023. CheckMate -901 exhibited that the Opdivo, cisplatin, and gemcitabine combination, followed by Opdivo monotherapy, significantly improved overall survival and progression-free survival compared to chemotherapy alone, based on the Blinded Independent Central Review's evaluation.
“In the CheckMate -901 research, combining Opdivo with cisplatin and gemcitabine boosted overall survival, reduced disease progression or death risk by 28% compared to chemotherapy alone, and showed more profound and lasting responses than chemotherapy alone,” said Michiel Van der Heijden, M.D, Ph.D., medical oncologist and research group leader at the Netherlands Cancer Institute.
The EC's approval of the combination of Opdivo with cisplatin and gemcitabine as a first-line treatment for adult patients with unresectable or metastatic UC applies to all 27 EU member states, alongside Iceland, Liechtenstein, and Norway.
Opdivo operates as a programmed death-1 (PD-1) immune checkpoint inhibitor, designed to leverage the body’s immune system to re-establish anti-tumor immunity effectively. By engaging the body's immune defenses to combat cancer, Opdivo has solidified its role as a vital treatment option across various cancers.
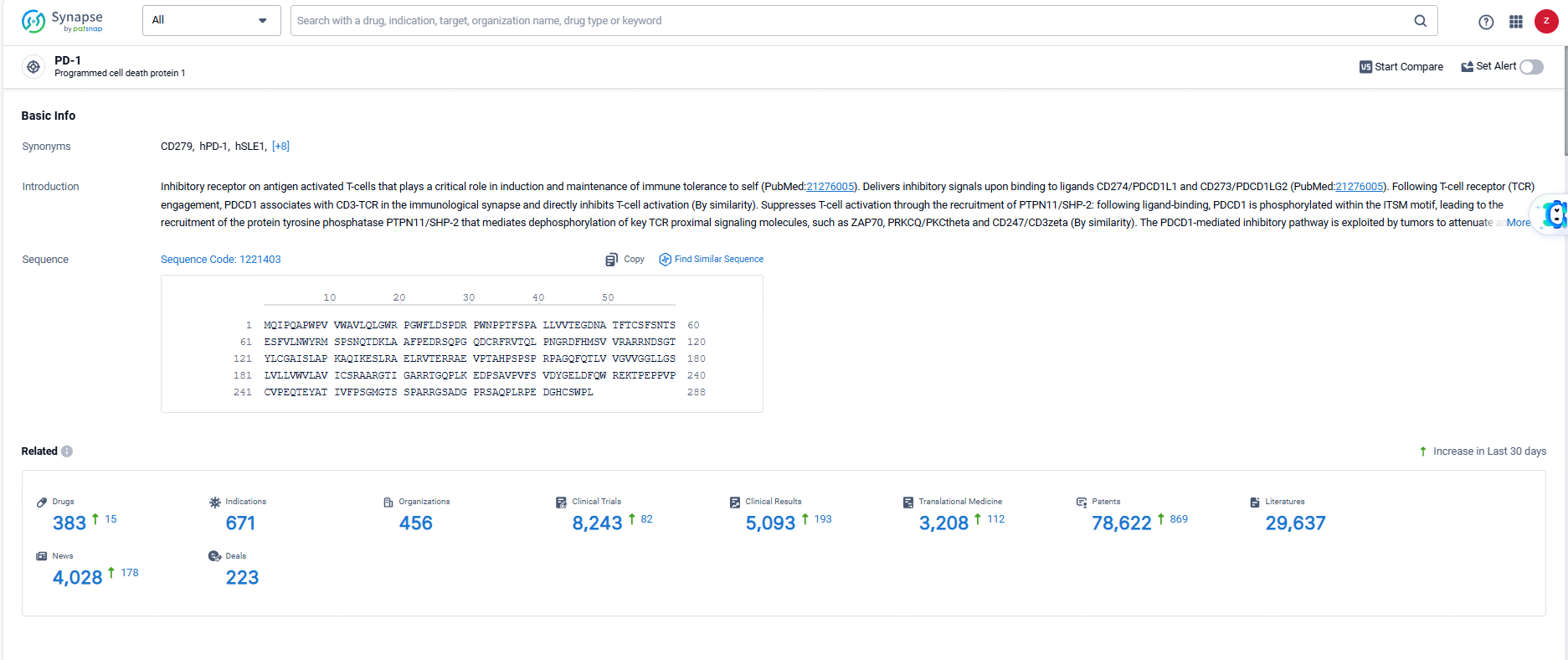
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 3, 2024, there are 383 investigational drugs for the PD-1 targets, including 671 indications, 456 R&D institutions involved, with related clinical trials reaching 8243, and as many as 29637 patents.
Nivolumab is a monoclonal antibody drug that targets PD-1 and has been approved for use in a wide range of therapeutic areas, including neoplasms, immune system diseases, digestive system disorders, respiratory diseases, and many others. The drug has been indicated for the treatment of various types of cancer, including skin neoplasms, urothelial carcinoma of the urinary bladder, small cell lung cancer, hepatocellular carcinoma, colorectal cancer, head and neck neoplasms, and many more.