Subcutaneous Nivolumab Plus Hyaluronidase as Effective as IV Opdivo for Advanced Kidney Cancer: CheckMate-67T Study
Bristol Myers Squibb has released initial findings from the CheckMate -67T Phase 3 study. The research assesses a combination of Opdivo (nivolumab) administered subcutaneously with Halozyme's unique recombinant human hyaluronidase PH20 enzyme, and compares its efficacy and safety with the intravenous version of Opdivo in individuals with advanced or metastatic clear cell renal cell carcinoma, who have been previously treated with systemic therapies. The outcomes indicated that for the main measures of average drug concentration over 28 days (Cavgd28) and steady-state minimum concentration (Cminss), the subcutaneous formulation met the noninferiority criteria compared with the intravenous administration of Opdivo.
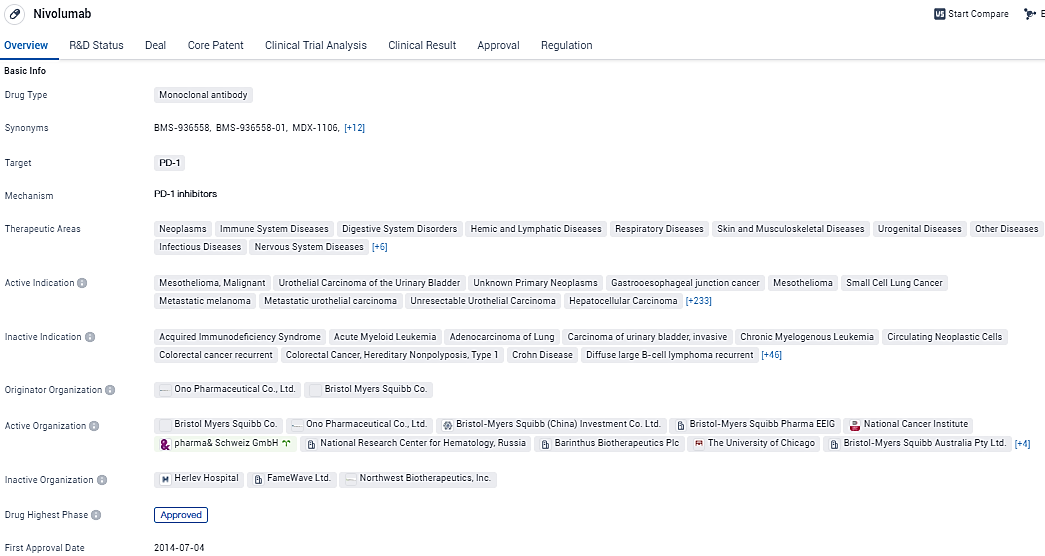
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Furthermore, the subdermal administration of nivolumab showcased an objective response rate on par with that of IV Opdivo, as evaluated by a Blinded Independent Central Review. These findings are set to be highlighted in a prominent late-breaking oral session at the annual American Society of Clinical Oncology 2024 Genitourinary Cancers Symposium, scheduled for January 25-27, 2024.
"The breakthroughs we've achieved with the subdermal nivolumab formula, which is combined with recombinant human hyaluronidase, mark a significant milestone in cancer research for medical professionals and those under our care," remarked Saby George, M.D., F.A.C.P., an authority in Oncology and Medicine, and the lead of Network Clinical Trials at the Department of Medicine within the Roswell Park Comprehensive Cancer Center.
Dr. George added, "The reality is, delivering immunotherapy through an IV can be time-consuming – a resource we know is critical for both patients and physicians. This is precisely why the demonstration of subdermal nivolumab's comparable efficacy is so crucial; it could revolutionize practice and enhance the overall treatment encounter for patients. This is done through a single injection that can be administered in less than five minutes and potentially performed outside of a traditional infusion clinic."
"The data emerging from the CheckMate -67T study reinforces our robust scientific foundation in applying immunotherapy to treat solid tumors and our dedication to enhancing the welfare of patients," expressed Gina Fusaro, Ph.D., Global Program Vice President at Bristol Myers Squibb. "We are excited to unveil this innovative research, offering the first-ever evaluation of subdermal nivolumab, which has shown promise in matching the efficacy of IV Opdivo and has the potential to refine the delivery of healthcare services."
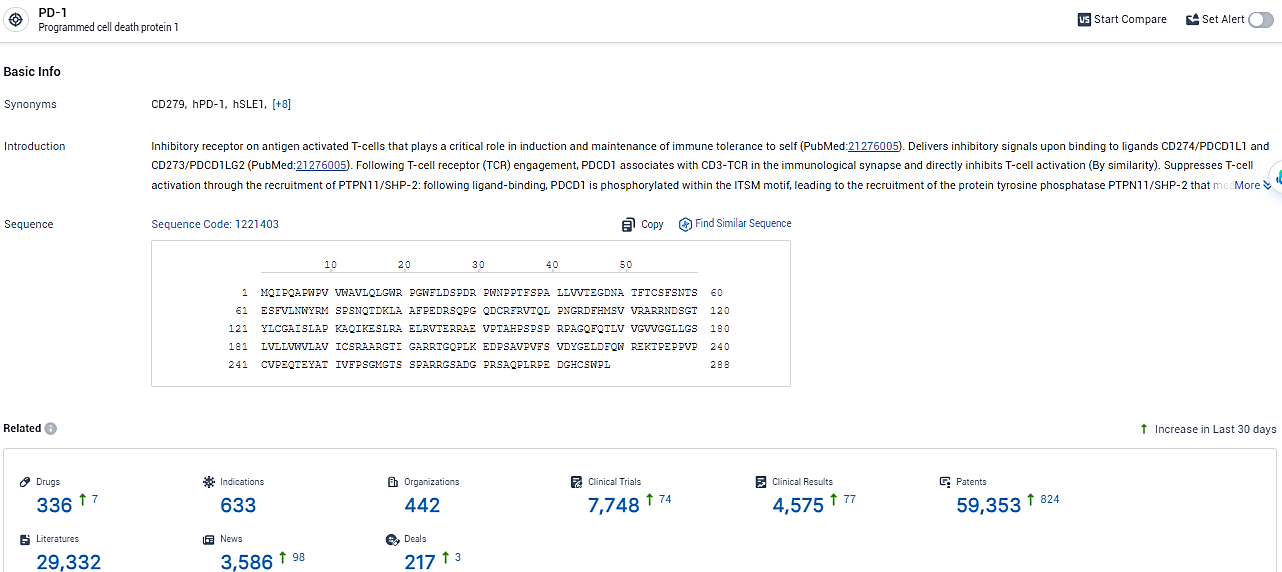
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 31, 2024, there are 336 investigational drugs for the PD-1 target, including 633 indications, 442 R&D institutions involved, with related clinical trials reaching 7748, and as many as 59353 patents.
Opdivo is a programmed death-1 (PD-1) immune checkpoint inhibitor that is designed to uniquely harness the body’s own immune system to help restore anti-tumor immune response. By harnessing the body’s own immune system to fight cancer, Opdivo has become an important treatment option across multiple cancers.