Successful Phase 1 Results for TERN-601, a Daily Oral GLP-1R Agonist in Obesity Treatment
Terns Pharmaceuticals, Inc. ("Terns" or the "Company") (Nasdaq: TERN), a clinical-stage biopharmaceutical organization focusing on developing a series of small-molecule product candidates for serious diseases, has revealed positive top-line results from its Phase 1 randomized, double-blind, placebo-controlled single and multiple-ascending dose (SAD and MAD) study. This trial aimed to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of once-daily (QD) administered TERN-601 in healthy adults who are either obese or overweight.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The findings from the clinical trial indicated that TERN-601 was well tolerated and showed a dose-responsive, significantly higher placebo-adjusted average weight loss across all three dosages examined in the 28-day MAD study. The highest placebo-adjusted average weight loss observed was 4.9% (p<0.0001) at the maximum dose of 740 mg QD. Furthermore, 67% of the participants achieved a reduction in their baseline body weight by 5% or more at the highest dose.
“These remarkable findings highlight TERN-601’s potential to become a leading GLP-1R agonist, given its preliminary signs of efficacy, tolerability, and potential for scalable manufacturing,” said Amy Burroughs, CEO of Terns. “This data substantiates the potential of TERN-601 for obesity treatment either as a stand-alone therapy or in combination with compounds like TERN-501, our internally developed, clinical-stage THR-β agonist, or a GIPR modulator from the TERN-800 series. With preparations actively in progress, we are excited to move this promising candidate swiftly into Phase 2 clinical trials by 2025.”
“We are pleased to showcase potent GLP-1R agonism with TERN-601, owing to its unique drug properties that allow sustained target engagement with once-daily dosing and evaluation of doses up to 740 mg, all while maintaining tolerability,” stated Emil Kuriakose, Chief Medical Officer of Terns. “Crucially, we believe we have identified an optimal range of clinically effective, well-tolerated doses to carry forward into Phase 2 clinical trials, with no need for further dose range exploration anticipated.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
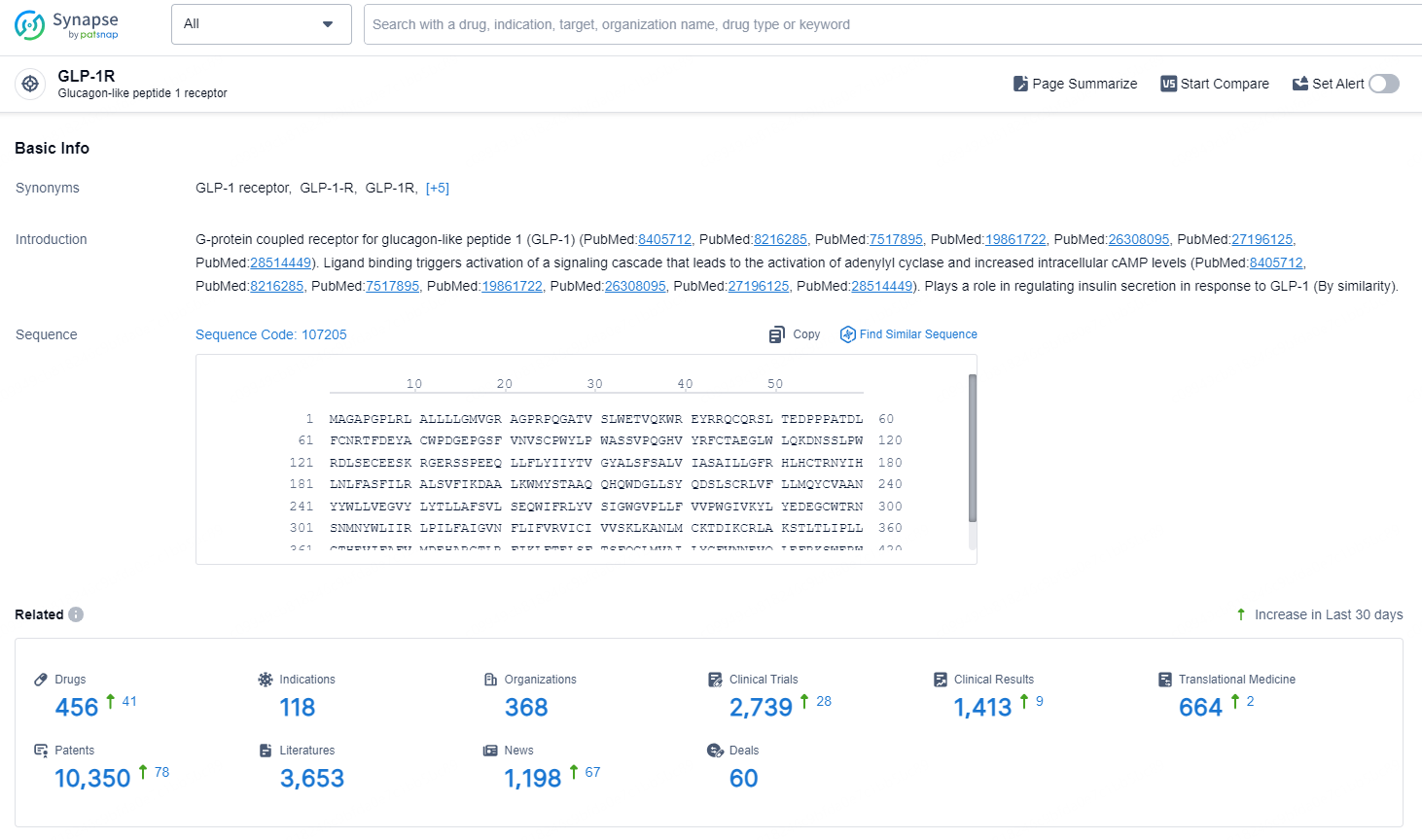
According to the data provided by the Synapse Database, As of September 11, 2024, there are 456 investigational drugs for the GLP-1R target, including 119 indications, 368 R&D institutions involved, with related clinical trials reaching 2739, and as many as 10350 patents.
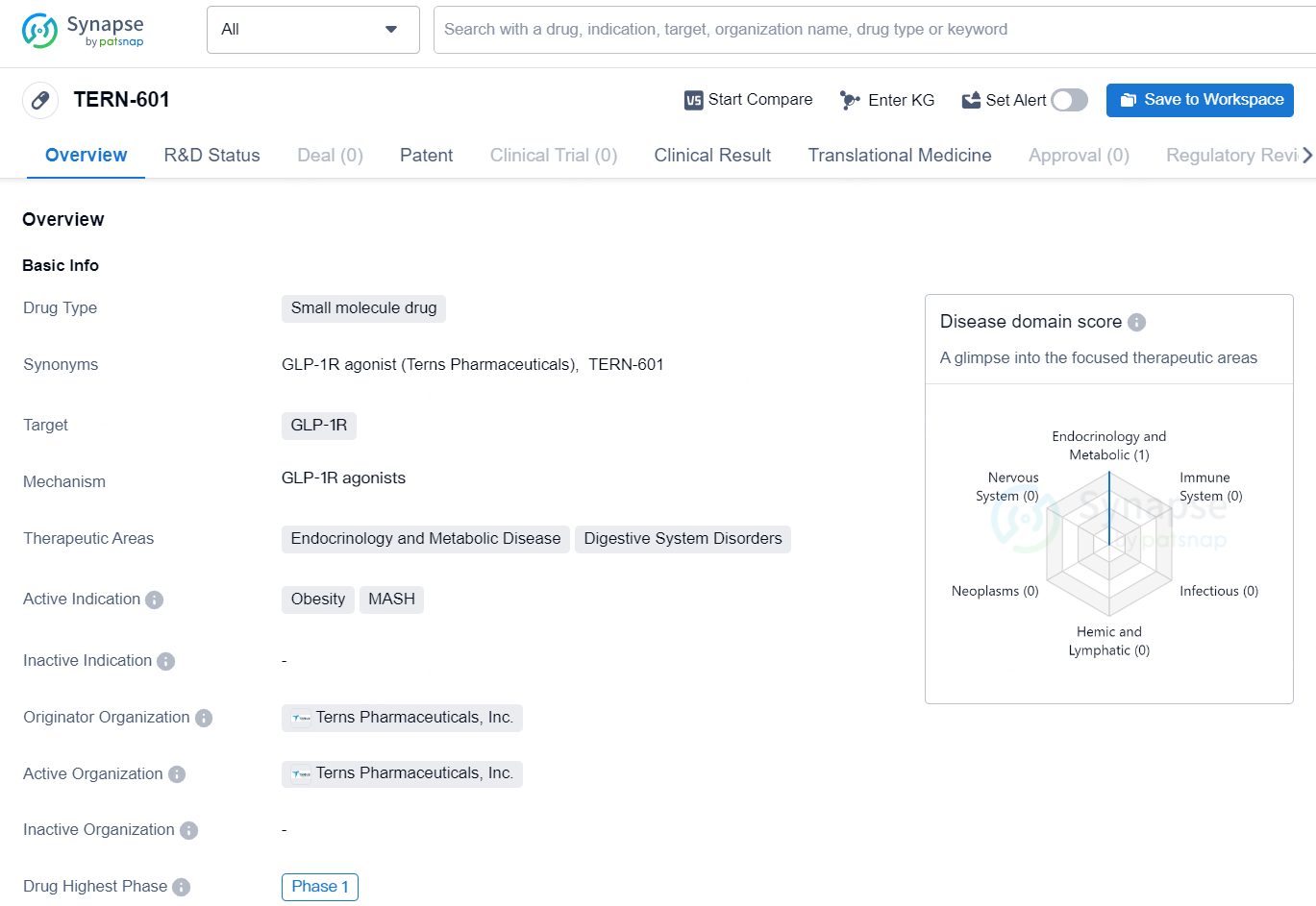
TERN-601 is a small molecule drug developed by Terns Pharmaceuticals, Inc. The drug targets GLP-1R and has shown potential therapeutic effects in the areas of endocrinology and metabolic diseases, as well as digestive system disorders. Its active indications include obesity and MASH (a condition that involves the combination of non-alcoholic fatty liver disease and steatohepatitis).