Tagrisso gains US approval for treating inoperable Stage III lung cancer with EGFR mutations
AstraZeneca’s Tagrisso (osimertinib) has received approval in the United States for treating adult patients with unresectable Stage III epidermal growth factor receptor-mutated (EGFRm) non-small cell lung cancer (NSCLC). This approval applies to patients whose condition has remained stable during or after concurrent or sequential platinum-based chemoradiation therapy (CRT). Tagrisso is intended for individuals who have exon 19 deletions or exon 21 (L858R) mutations, verified by an FDA-approved test.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Food and Drug Administration (FDA) has granted approval after a Priority Review, driven by data from the LAURA Phase III trial. The findings from this study were showcased at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting's Plenary Session and concurrently published in The New England Journal of Medicine.
Tagrisso demonstrated an 84% reduction in the risk of disease progression or death compared to placebo (hazard ratio 0.16; 95% confidence interval 0.10-0.24; p<0.001), per a blinded independent central review. The median progression-free survival (PFS) was 39.1 months for patients on Tagrisso, against 5.6 months for those on placebo.
The overall survival (OS) data are still considered immature at this stage of analysis, with the trial ongoing to evaluate OS as a secondary endpoint.
In the United States, over 200,000 individuals are diagnosed with lung cancer annually, with 80-85% of these cases being non-small cell lung cancer (NSCLC). Approximately 15% of NSCLC patients in the US have EGFR mutations, and nearly 20% of NSCLC cases involve unresectable tumors.
Dr. Suresh Ramalingam, Executive Director at Winship Cancer Institute of Emory University and the principal investigator of the trial, commented: “This approval signifies a significant advancement for patients with Stage III, EGFR-mutated lung cancer, who will now have access to the benefits of osimertinib. In the LAURA trial, patients treated with osimertinib experienced over three years without disease progression, highlighting the critical need for early diagnosis and testing in lung cancer.”
Dave Fredrickson, Executive Vice President of the Oncology Business Unit at AstraZeneca, added: “The approval of Tagrisso for patients with Stage III, unresectable EGFR-mutated NSCLC meets a crucial need for patients who previously lacked targeted therapy options. The LAURA trial results underline Tagrisso’s potential as a cornerstone in the treatment of this disease, and with this approval, patients across all stages of EGFR-mutated NSCLC can now benefit.”
The safety and tolerability profile of Tagrisso in the LAURA trial aligned with its known profile, with no new safety concerns identified.
Tagrisso is approved for patients with EGFR mutations in the first-line metastatic setting, both as a monotherapy and in combination with chemotherapy, as well as an adjuvant treatment for early-stage disease. It is currently under regulatory review in other countries worldwide for this indication.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
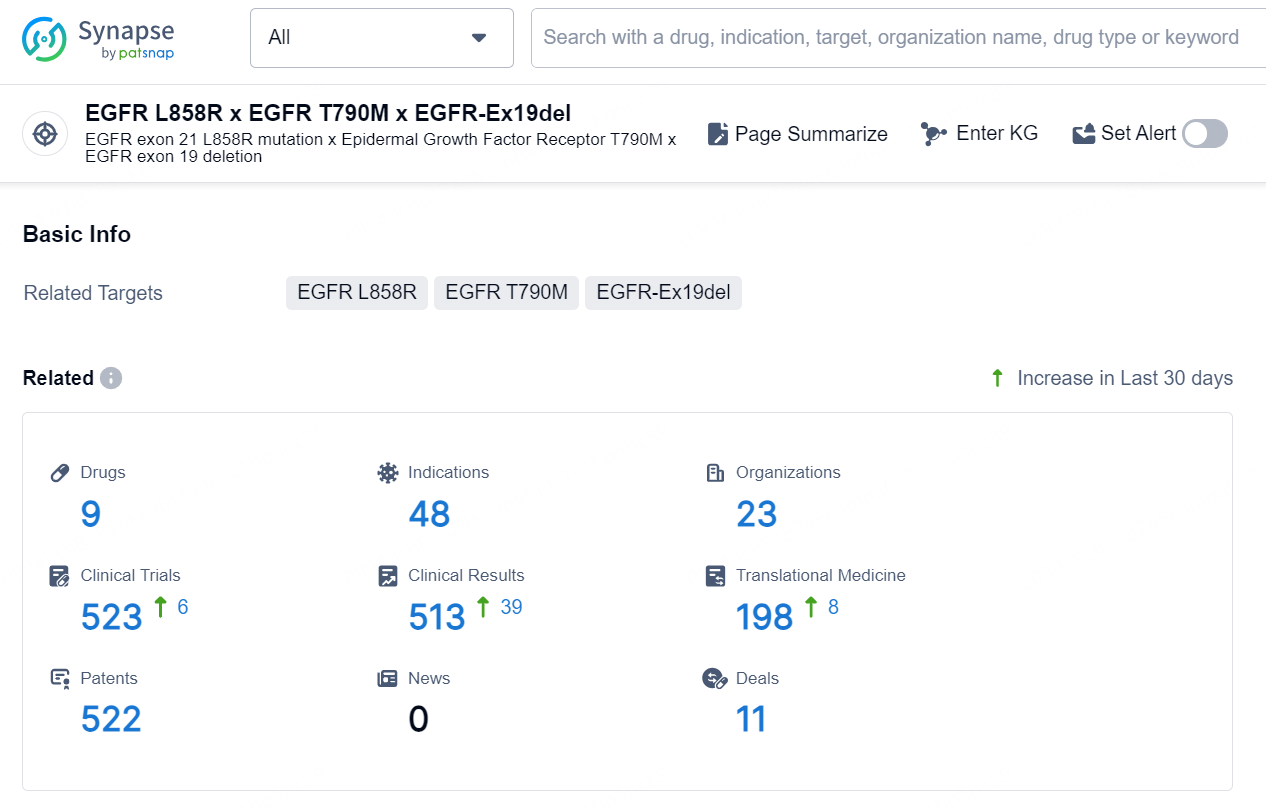
According to the data provided by the Synapse Database, As of September 29, 2024, there are 9 investigational drug for the EGFR L858R x EGFR-Ex19del x EGFR T790M targets, including 48 indications, 23 R&D institutions involved, with related clinical trials reaching 523, and as many as 522 patents.
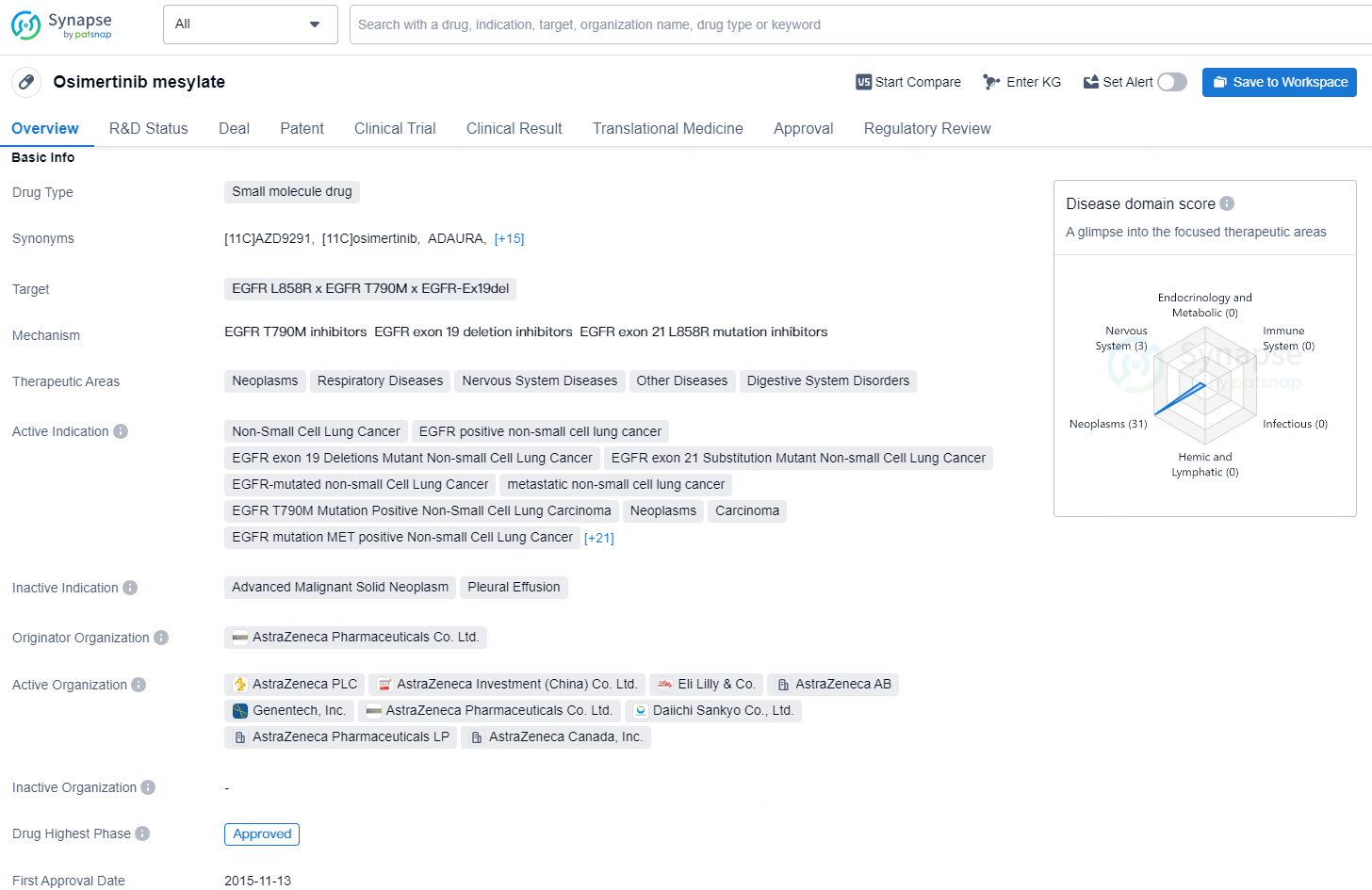
Osimertinib mesylate is a small molecule drug developed by AstraZeneca Pharmaceuticals Co. Ltd. It targets the EGFR L858R, EGFR T790M, and EGFR-Ex19del mutations and is indicated for the treatment of various types of neoplasms, respiratory diseases, nervous system diseases, digestive system disorders, and other diseases. The drug has been approved for a wide range of indications, including non-small cell lung cancer (NSCLC), EGFR-positive NSCLC, EGFR exon 19 deletions mutant NSCLC, EGFR exon 21 substitution mutant NSCLC, EGFR-mutated NSCLC, metastatic NSCLC, EGFR T790M mutation positive NSCLC, resectable and unresectable NSCLC, second primary neoplasms, locally advanced NSCLC, EGFR ex20ins mutation in NSCLC, brain metastases, recurrent glioblastoma, stage I, II, and III NSCLC, advanced and refractory lung small cell carcinoma, metastatic colorectal carcinoma, and solid tumors.