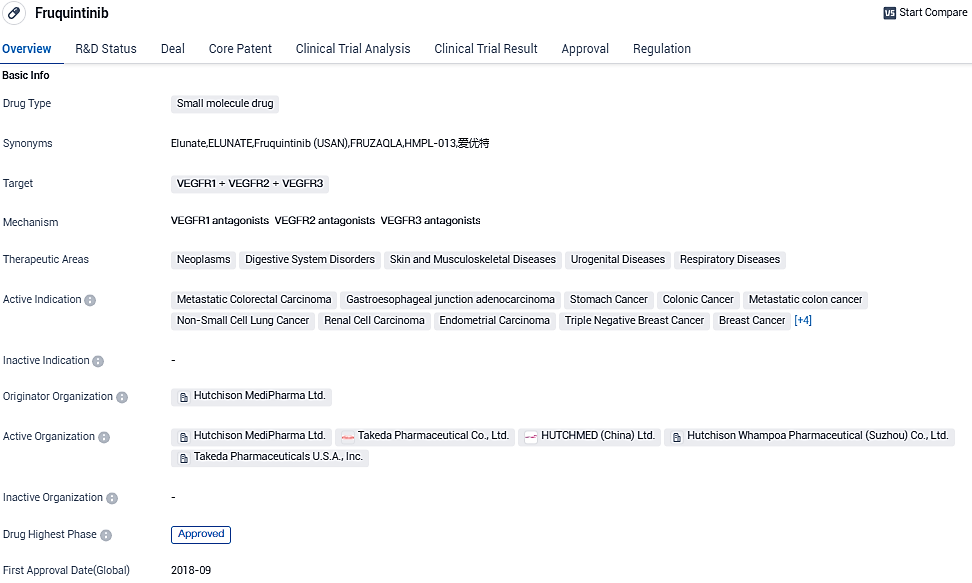
Takeda Gets Green Light from U.S. FDA to Market FRUZAQLA™ (fruquintinib) for a Pre-treated Type of Advanced Colon Cancer
Takeda has declared that FRUZAQLA™ (fruquintinib), a targeted oral treatment, has received FDA approval. This treatment is intended for adults suffering from metastatic colorectal cancer who have earlier undergone chemotherapy based on fluoropyrimidine, oxaliplatin, and irinotecan, an anti-VEGF treatment, as well as an anti-EGFR treatment if RAS is wild-type and clinically applicable.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
FRUZAQLA represents the first and sole selective inhibitor of three VEGF receptor kinases given the green light in the U.S. for the treatment of metastatic colorectal cancer (mCRC) previously treated, regardless of biomarker status. This authorization was granted under Priority Review, over 20 days ahead of the planned PDUFA date of November 30, 2023.
"There is an urgent need for innovative therapies for individuals diagnosed with metastatic colorectal cancer who have limited alternatives and continue to contend with less than optimal outcomes. FRUZAQLA, the first new non-chemotherapy-based treatment approach approved in the U.S. for patients regardless of their biomarker status in over ten years, fills this need," asserted Teresa Bitetti, Leader of the Global Oncology Business Unit at Takeda.
Bitetti further commented, "There has been a critical need for diverse treatment options for healthcare providers and patients in their search for therapies for metastatic colorectal cancer. FRUZAQLA has the capability to provide an extraordinary survival advantage to patients, without adversely affecting their quality of life."
Approximately 153,000 new instances of CRC will be identified in the United States in 2023, accounting for roughly 7.8% of all newfound cancer instances. Almost 70% of those diagnosed with CRC will display metastatic disease, whether at the time of diagnosis or subsequent treatment. Metastases account for the leading cause of CRC-related deaths.
Metastatic colorectal cancer inflicts both physical and emotional distress on patients, their families and their caregivers, which we have observed firsthand," shared Michael Sapienza, CEO at Colorectal Cancer Alliance. "It is heartening to see the ongoing progress in the development of new treatments for such patients."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
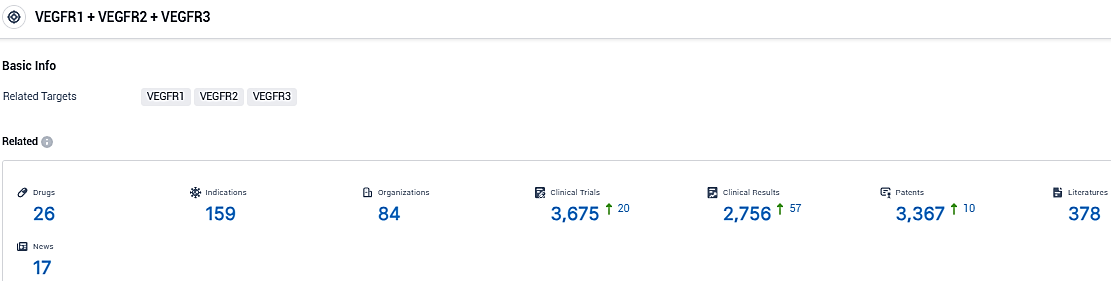
According to the data provided by the Synapse Database, As of November 16, 2023, there are 26 investigational drugs for the VEGFR1 and VEGFR2 and VEGFR3 target, including 159 indications, 84 R&D institutions involved, with related clinical trials reaching 3675, and as many as 3367 patents.
Fruquintinib was approved for marketing by NMPA in September 2018 and commercially launched in China in November 2018 under the brand name ELUNATE®.Takeda has the exclusive worldwide license to further develop, commercialize, and manufacture fruquintinib outside of mainland China, Hong Kong and Macau. Fruquintinib is developed and marketed in China by HUTCHMED.