The EU approves VANFLYTA®, a new FLT3 inhibitor for FLT3-ITD positive AML patients
The European Union has given the green light for the application of Daiichi Sankyo's VANFLYTA (quizartinib). It is to be used alongside the standard cytarabine and anthracycline induction, as well as the standard cytarabine consolidation chemotherapy. After these therapies, VANFLYTA is to be used individually as a maintenance treatment. This treatment regimen is specifically for adult patients who have been recently diagnosed with acute myeloid leukemia, and whose condition tests positive for FLT3-ITD.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
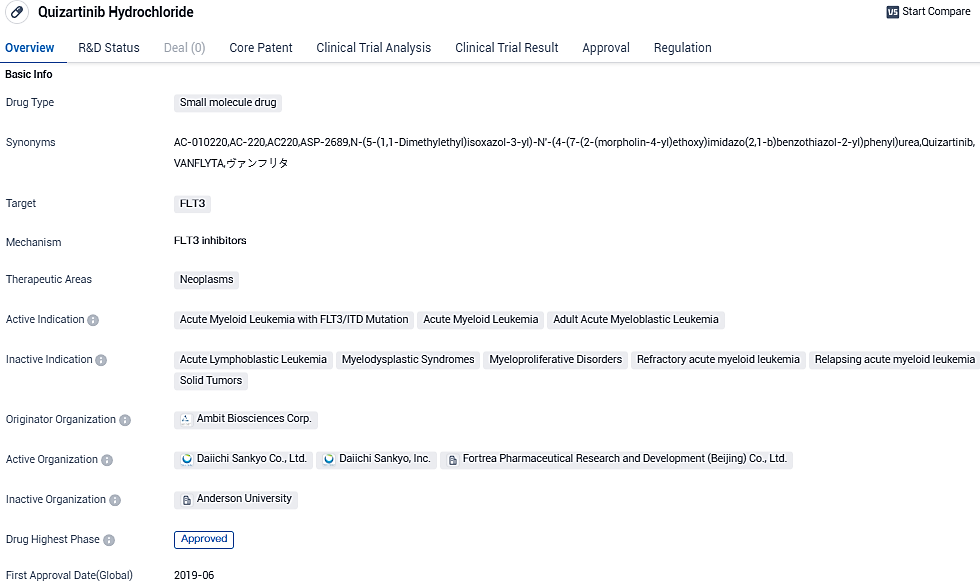
VANFLYTA, the first FLT3 inhibitor cleared for use in the EU, is designed expressly for treating newly diagnosed FLT3-ITD positive AML patients, accounting for around 25 to 30% of all new AML cases. The European Commission's approval stemmed from the positive reception of the Committee for Medicinal Products for Human Use and is rooted in the findings of the QuANTUM-First study, seen in The Lancet. In this trial, VANFLYTA, supplemented with standard cytarabine and anthracycline induction and standard cytarabine consolidation, and used as ongoing maintenance monotherapy after consolidation, exhibited a 22% drop in the death risk versus just standard chemotherapy in patients with newly diagnosed FLT3-ITD positive AML.
Richard F. Schlenk, MD, Professor and Director of the Trial Center at the National Center of Tumour Diseases, Heidelberg University Hospital and German Cancer Research Center remarked, "This authorization of VANFLYTA is an essential step forward in first-line treating patients suffering from FLT3-ITD positive acute myeloid leukemia, a historically challenging and aggressive subtype to manage."
He further added, "VANFLYTA, a powerful, selective FLT3 inhibitor, significantly boosts total survival when incorporated into standard chemotherapy, and it adds an effective treatment alternative for those newly diagnosed with FLT3-ITD positive AML."
Acute Leukemia Advocates Network's Network Director, Samantha Nier, added, "The favorable decision on VANFLYTA is brilliant news for patients qualified and diagnosed annually with FLT3-ITD positive AML. This difficult leukemia type needs new medication and treatment methods to help patients live longer and we're eagerly awaiting VANFLYTA being accessible in countries across the EU."
"With the clearance for VANFLYTA's use in the European Union, for the first time, patients with newly diagnosed FLT3-ITD positive acute myeloid leukemia can benefit from targeted therapy, specifically evolved and sanctioned for their disease subtype," commented Ken Keller, Global Chief of Oncology Business, as well as President and CEO of Daiichi Sankyo, Inc. "VANFLYTA is the second pioneering drug approved in the EU from our oncology spectrum, emphasizing our dedication to establishing new care standards for cancer patients."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
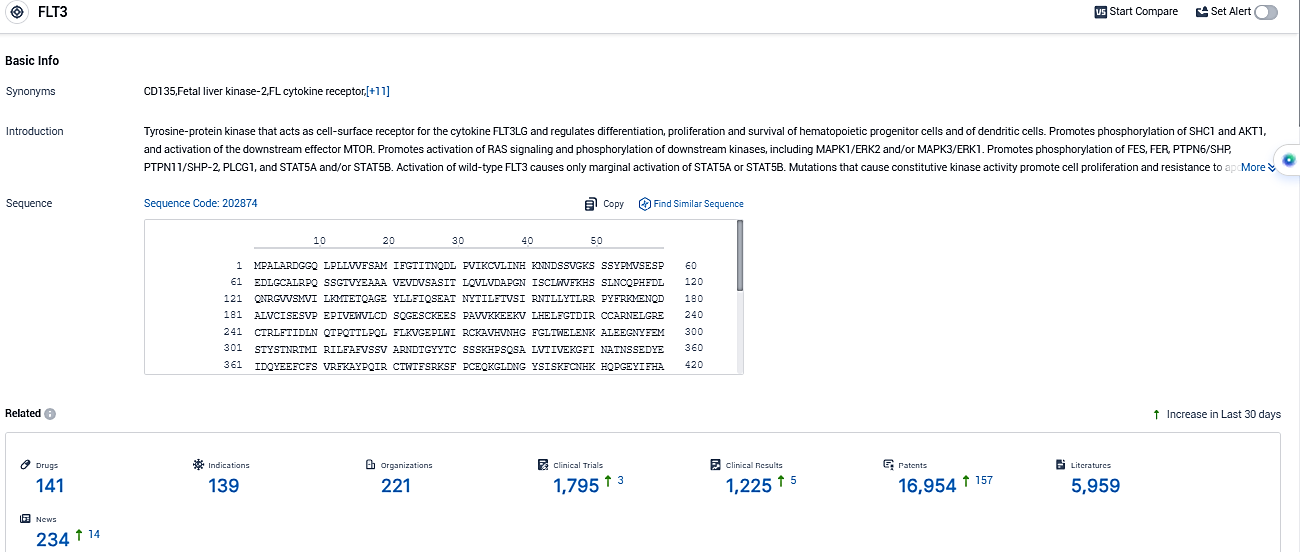
According to the data provided by the Synapse Database, As of November 16, 2023, there are 141 investigational drugs for the FLT3 target, including 139 indications, 221 R&D institutions involved, with related clinical trials reaching 1795, and as many as 16954 patents.
VANFLYTA is approved in the EU in combination with standard cytarabine and anthracycline induction and standard cytarabine consolidation chemotherapy, followed by VANFLYTA single-agent maintenance therapy for adult patients with newly diagnosed AML that is FLT3-ITD positive.