Takeda's ADZYNMA Clears EU Commission for cTTP Treatment
Takeda revealed that the European Commission has granted approval for ADZYNMA®▼ (recombinant ADAMTS13) to be utilized in the management of ADAMTS13 deficiency in both pediatric and adult individuals suffering from congenital thrombotic thrombocytopenic purpura.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 ADZYNMA has become the first and only enzyme replacement therapy in the European Union specifically approved for the treatment of cTTP. This approval includes the confirmation of its orphan medicinal product designation and follows a favorable opinion from the Committee for Medicinal Products for Human Use, as stated by the company on May 31, 2024.
ADZYNMA has become the first and only enzyme replacement therapy in the European Union specifically approved for the treatment of cTTP. This approval includes the confirmation of its orphan medicinal product designation and follows a favorable opinion from the Committee for Medicinal Products for Human Use, as stated by the company on May 31, 2024.
cTTP, or congenital thrombotic thrombocytopenic purpura, is an extremely rare and chronic blood clotting disorder caused by a deficiency of the ADAMTS13 enzyme. This condition can lead to severe acute events and chronic symptoms including thrombocytopenia, microangiopathic hemolytic anemia, renal complications, stroke, and abdominal pain. If acute TTP events are not treated, they have a mortality rate exceeding 90%.
"Despite the scientific discovery of cTTP a century ago, significant unmet needs persist for patients who endure life-threatening acute events and chronic symptoms with limited treatment options," said Ricardo Marek, President of the Europe and Canada Business Unit at Takeda.
"This approval is a significant milestone as it introduces the first treatment specifically aimed at addressing the root cause of the disease—ADAMTS13 deficiency. Building upon our 70-year legacy of innovation in rare blood disorders, we are proud to offer ADZYNMA to cTTP patients in the EU. We are committed to delivering innovative medicines to patients with rare diseases who have high unmet needs," Ricardo Marek added.
The European Commission’s approval was based on a comprehensive review of the interim analysis of efficacy, pharmacokinetic, safety, and tolerability data from the first randomized, controlled open-label, crossover Phase 3 trial in cTTP, along with safety and efficacy data from the continuation trial.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
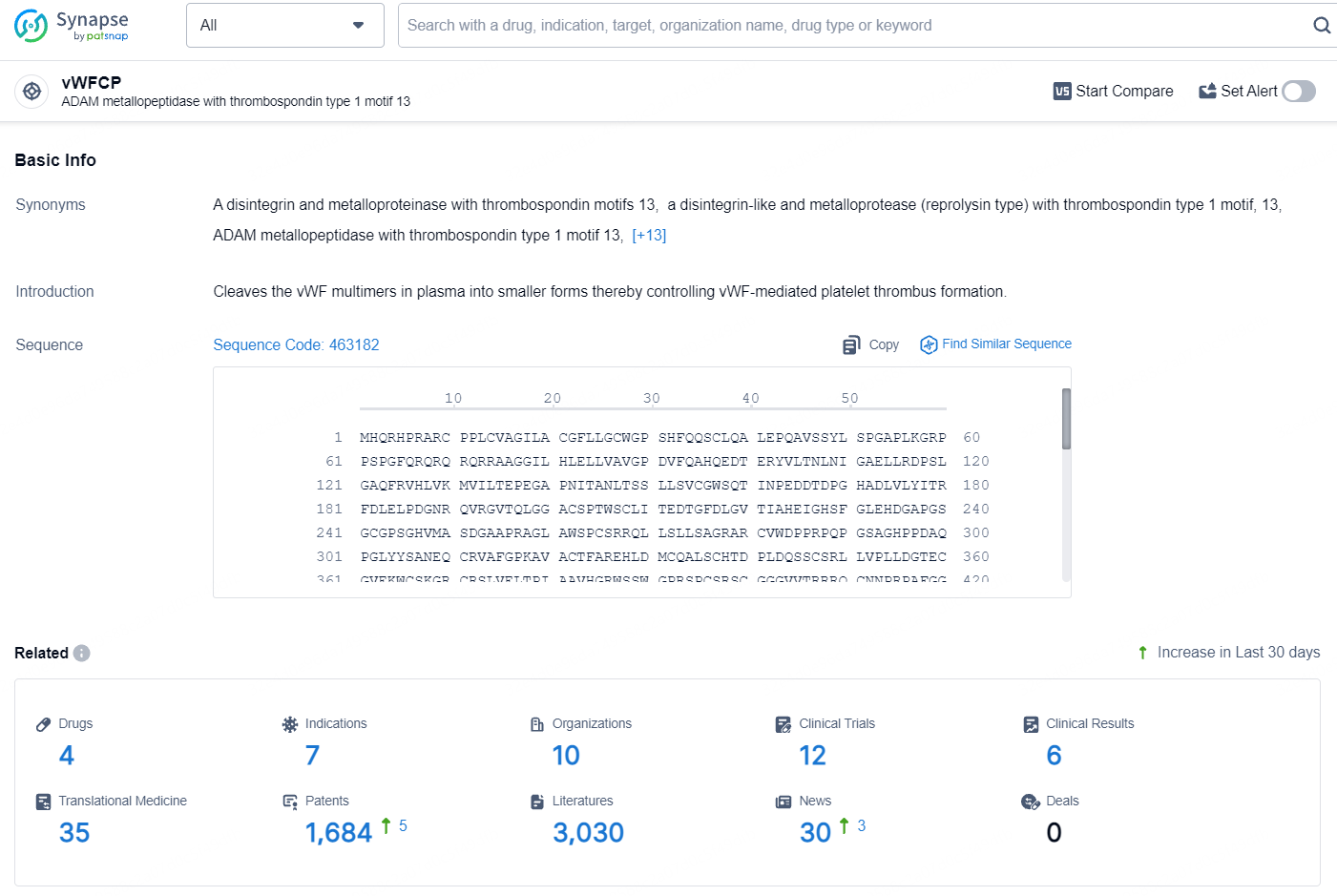
According to the data provided by the Synapse Database, As of August 12, 2024, there are 4 investigational drugs for the vWFCP target, including 7 indications, 10 R&D institutions involved, with related clinical trials reaching 12, and as many as 1684 patents.
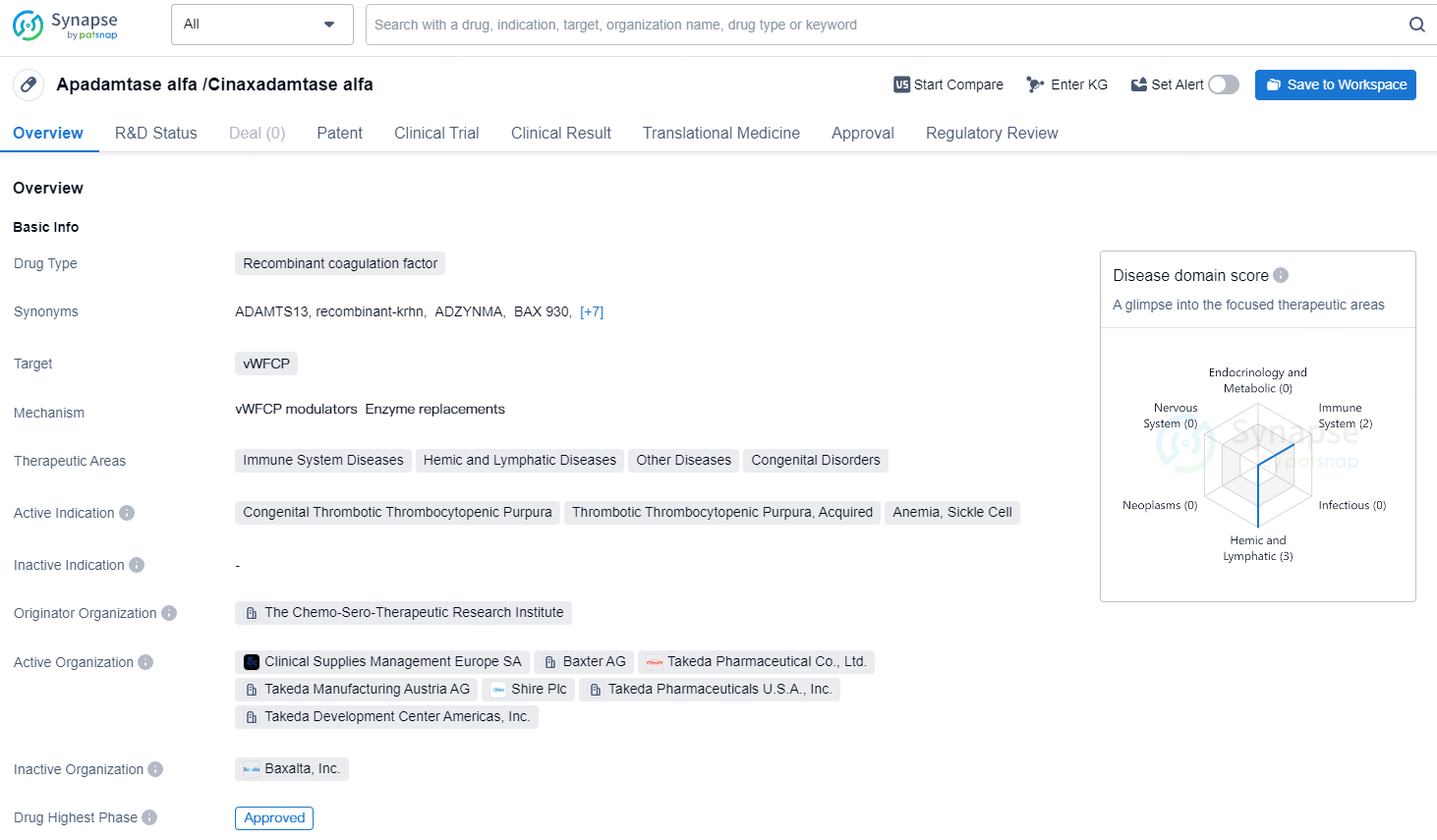
Recombinant ADAMTS13 alfa is a recombinant coagulation factor drug that targets vWFCP and is used in the treatment of various therapeutic areas including Immune System Diseases, Hemic and Lymphatic Diseases, Other Diseases, and Congenital Disorders. It has received approval in the United States, with potential approval in China. The drug has been designated with special regulatory statuses that could potentially expedite its market entry and provide market exclusivity.