Targeting Nectin-4: The Rise of Enfortumab Vedotin in ADC Cancer Therapy
On the cutting edge of cancer treatment, scientists are continually searching for new weapons capable of precisely targeting tumor cells without harming normal tissues. In recent years, a molecular target called Nectin-4 has emerged as a rising star in the field of anti-cancer drug development. Nectin-4 is a type I transmembrane protein that is minimally expressed in normal adult tissues but significantly overexpressed in various malignancies, such as urothelial carcinoma, breast cancer, pancreatic cancer, triple-negative breast cancer, and bladder cancer. This unique expression profile makes it an ideal candidate for the development of novel cancer therapies.
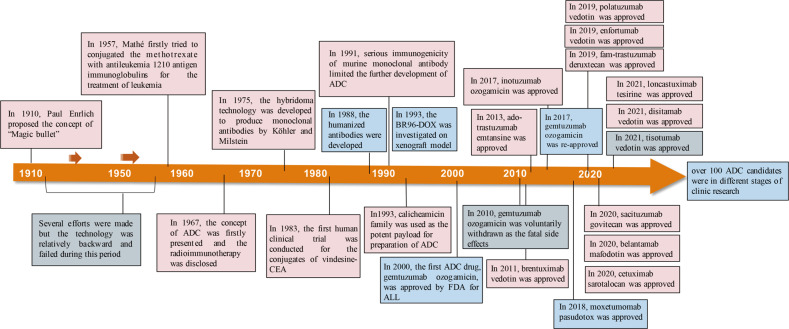
Notably, Nectin-4 is overexpressed in nearly all bladder cancers, providing a solid biological foundation for antibody-drug conjugates (ADCs) targeting this molecule. Enfortumab vedotin (brand name: Padcev), developed jointly by Seagen and Astellas Pharma, is the first ADC targeting Nectin-4 to gain approval from the U.S. Food and Drug Administration (FDA). This drug has not only validated the feasibility of targeting Nectin-4 for cancer therapy but has also demonstrated remarkable efficacy in clinical trials, heralding a new chapter for Nectin-4 as an anti-cancer target.
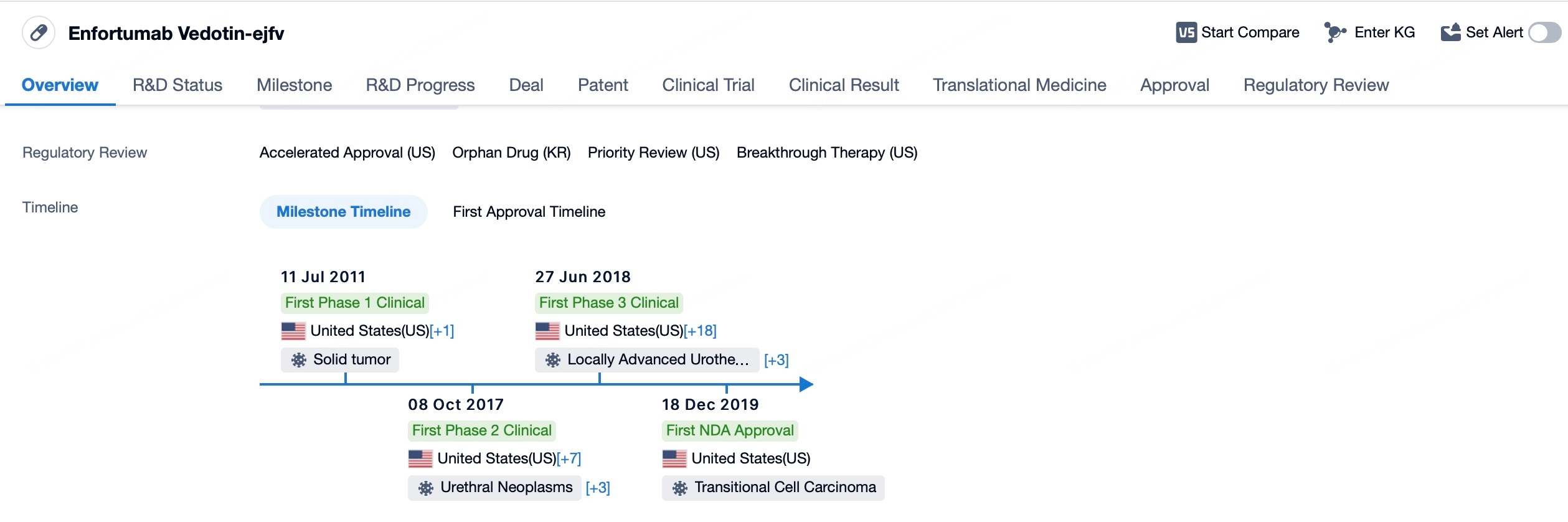
Since its accelerated FDA approval in December 2019 in the United States, Enfortumab vedotin has become the first ADC targeting Nectin-4, offering a new treatment option for patients with locally advanced or metastatic urothelial carcinoma. Subsequently, the efficacy and safety of Enfortumab vedotin have gained further recognition worldwide.
In the European Union, the drug was approved by the European Medicines Agency (EMA), broadening its accessibility in the European market. Similarly, Enfortumab vedotin has been approved in Canada and Australia. In August 2024, the National Medical Products Administration (NMPA) of China approved Enfortumab vedotin based on its promising clinical trial results for treating adult patients with locally advanced or metastatic urothelial carcinoma who had previously received PD-1/PD-L1 inhibitors and experienced disease progression during or after such treatments.
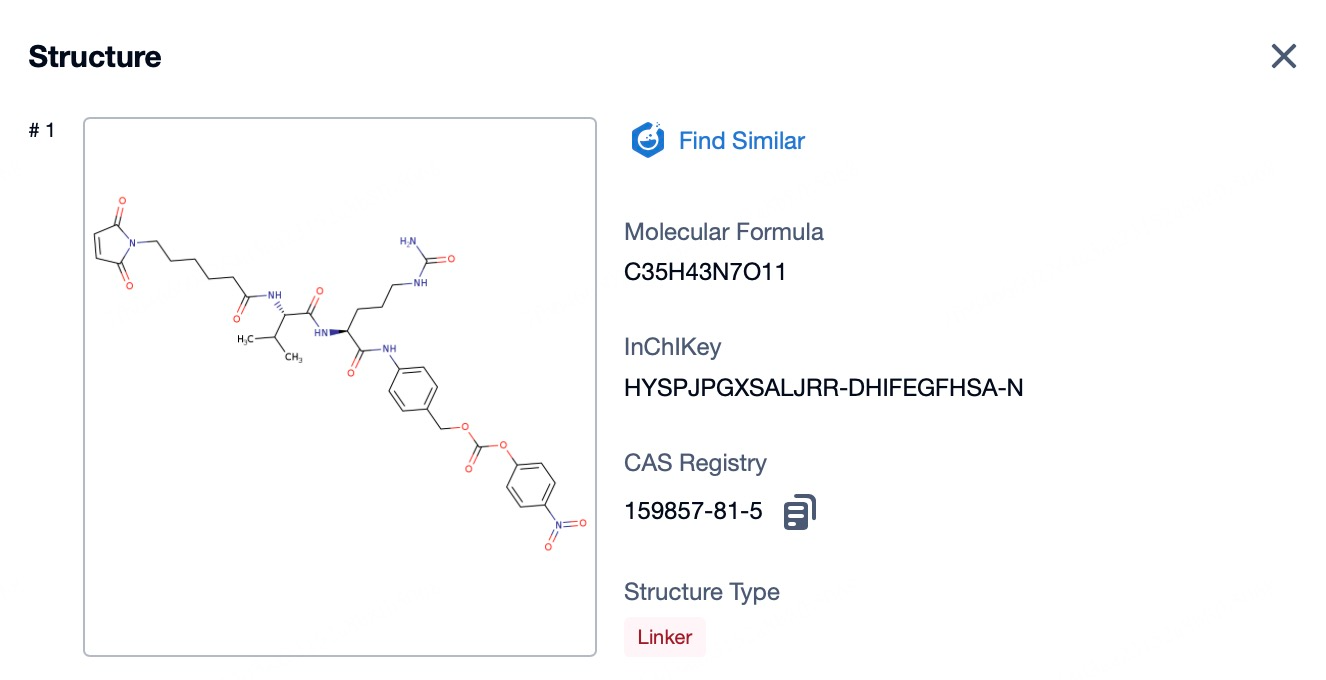
Linker in Enfortumab Vedotin
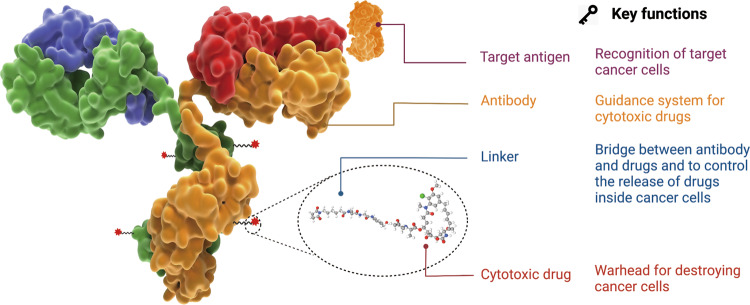
The linker plays a critical role in antibody-drug conjugates (ADCs), connecting the antibody with the cytotoxic payload while influencing the stability and release characteristics of the payload. An ideal linker design avoids nonspecific aggregation, ensures stability in plasma, and allows selective release of the payload at the target tumor site, optimizing therapeutic outcomes. Enfortumab vedotin leverages ADC linker technology developed by Seagen. This technology ensures the stability of the cytotoxic drug during delivery to tumor cells and its efficient release under specific conditions.
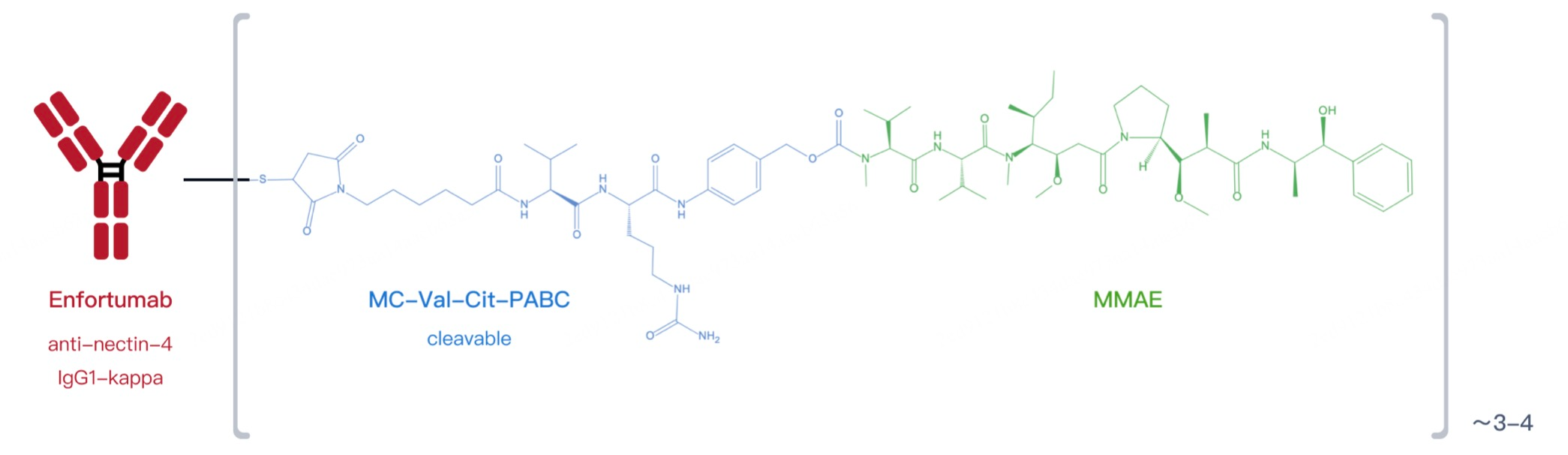
Seagen’s linker design is based on a valine-citrulline dipeptide structure. Upon reduction of interchain disulfide bonds in the IgG1 antibody, reactive thiol groups interact with active groups like maleimide on the linker, forming stable conjugates. In IgG1 antibodies, this process involves the reduction of four pairs of interchain disulfide bonds, producing eight reactive cysteine thiol groups, providing multiple conjugation sites. This design enhances ADC stability during production and minimizes nonspecific payload release in systemic circulation. Furthermore, Seagen's linker technology balances stability with controlled release, enabling the payload to be released under tumor-specific conditions, such as the intracellular environment, ensuring selective cytotoxicity to target cells while minimizing off-target effects.
Monoclonal Antibody in Enfortumab Vedotin
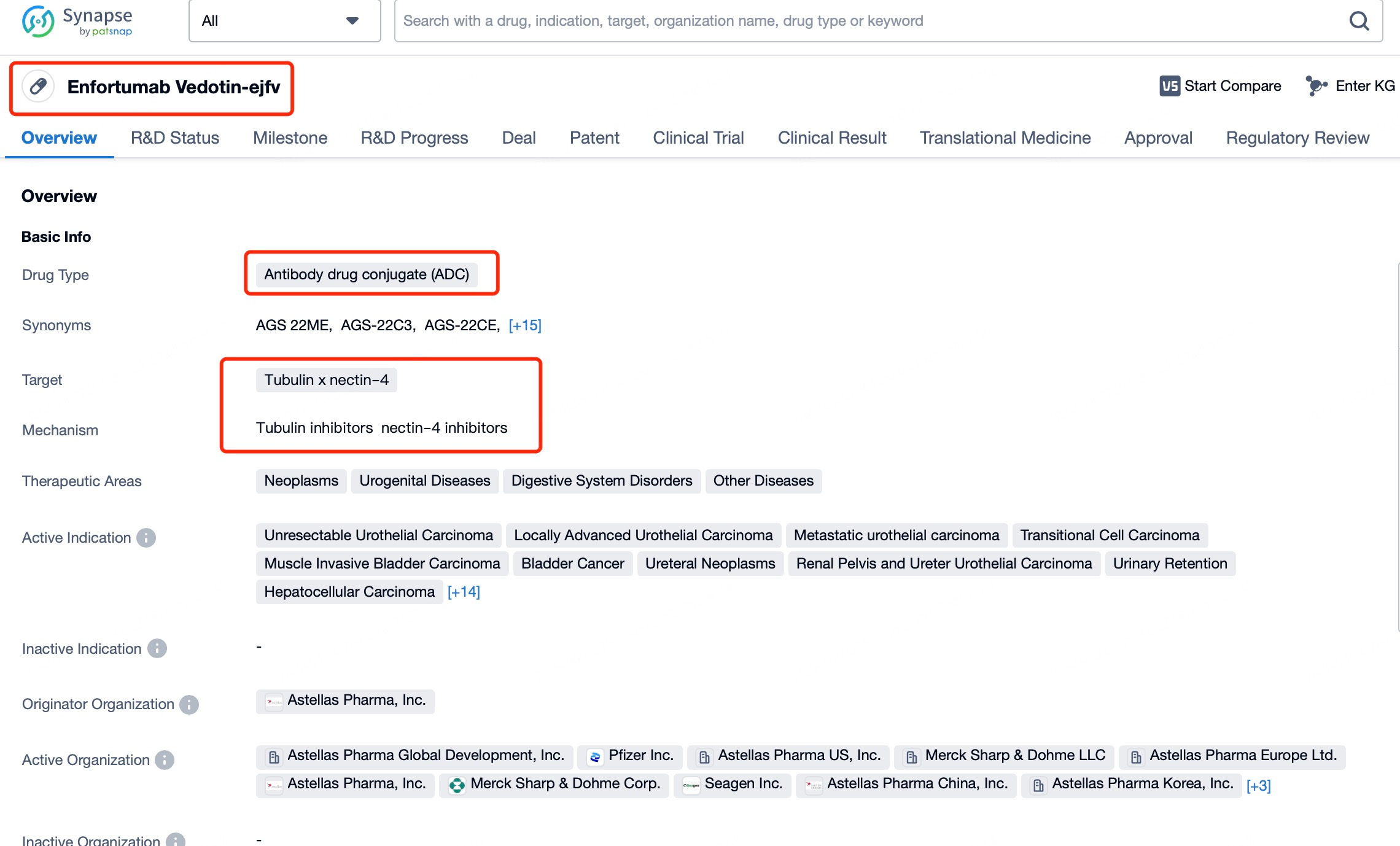
Astellas played a pivotal role in target identification, focusing on the therapeutic potential of Nectin-4. Also known as PVRL4, Nectin-4 is a transmembrane cell adhesion molecule of the Nectins family, highly expressed in urothelial carcinoma. It is a type I transmembrane protein (~66 kDa) encoded by the NECTIN4 gene on chromosome 1q23.3. Structurally, its extracellular domain contains three conserved Ig-like loops (one IgV and two IgC loops), while its cytoplasmic domain includes an afadin-binding motif. Nectin-4 is primarily expressed during embryonic and fetal development, with minimal expression in adult tissues. Astellas identified its high expression in urothelial carcinoma, establishing its viability as a therapeutic target for ADCs like Enfortumab vedotin.
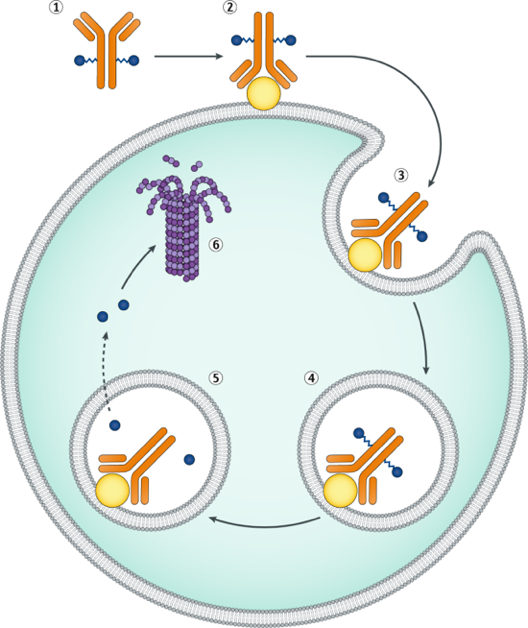
Enfortumab vedotin capitalizes on this characteristic by using a humanized IgG1 monoclonal antibody (Enfortumab) that specifically binds to Nectin-4 on tumor cell surfaces. This targeted binding not only precisely marks tumor cells but also facilitates efficient drug delivery. Enfortumab vedotin delivers the microtubule inhibitor monomethyl auristatin E (MMAE) directly into tumor cells, exerting antitumor activity within the tumor microenvironment. By inhibiting tumor cell proliferation and inducing apoptosis, Enfortumab vedotin provides a novel treatment option for patients.
Through Patsnap Bio, the antibody sequences of Enfortumab vedotin, including its critical complementarity-determining regions (CDRs) and constant (Fc) regions, can be thoroughly retrieved and analyzed.
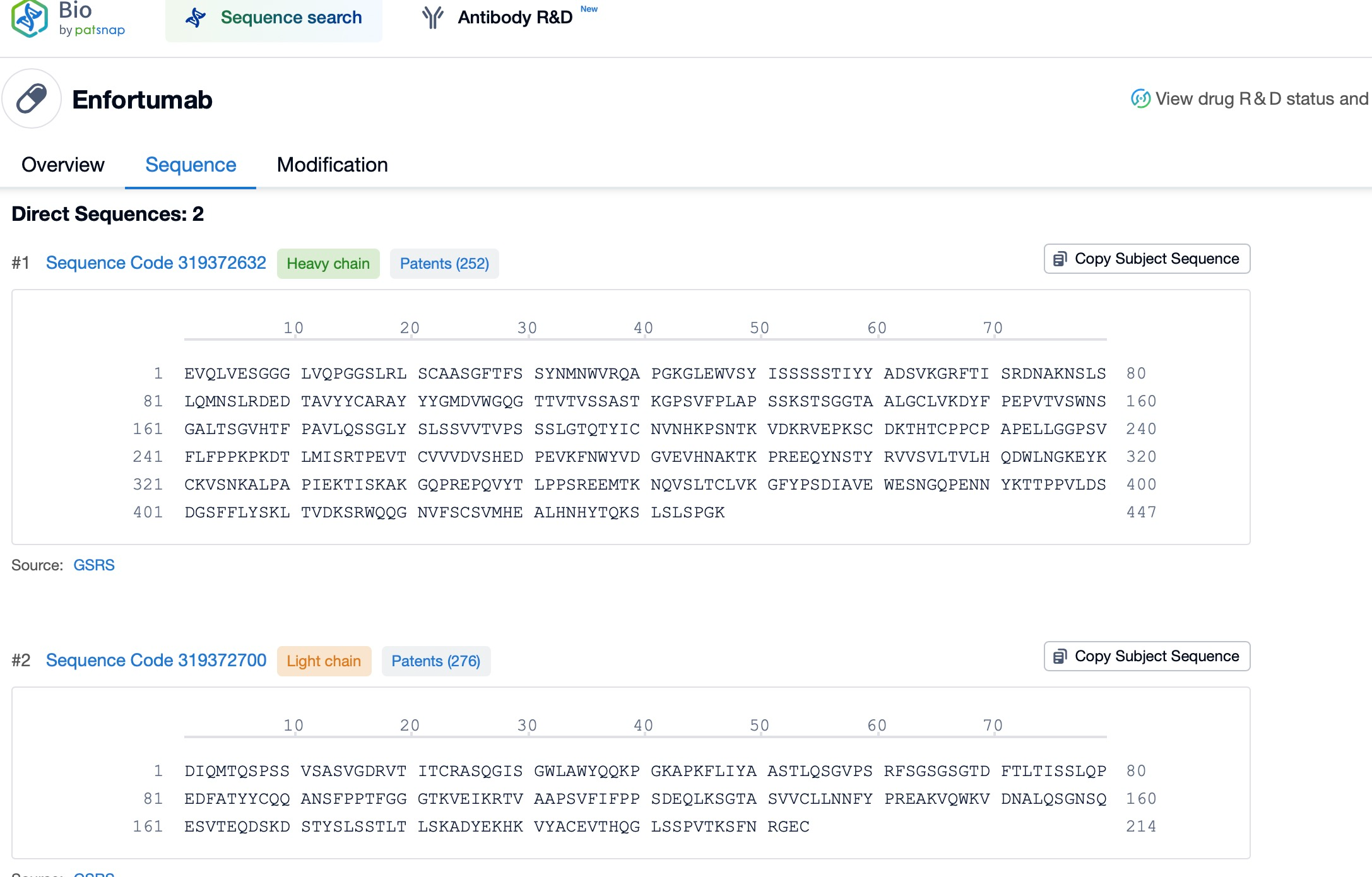
Detailed investigation of the CDR sequences allows for a deeper understanding of the binding mechanisms between the antibody and the Nectin-4 target. This involves identifying key amino acid residues essential for antibody affinity and stability. Such insights enable optimization of these regions via mutation or recombinant techniques, enhancing the antibody's performance. Additionally, this analysis helps assess the degree of humanization of the antibody, a crucial factor in reducing immunogenicity risk and improving tolerability in clinical applications. This data provides valuable guidance for designing novel antibody drugs, particularly ADCs targeting Nectin-4 or related antigens, ensuring scientifically robust and forward-looking drug development.
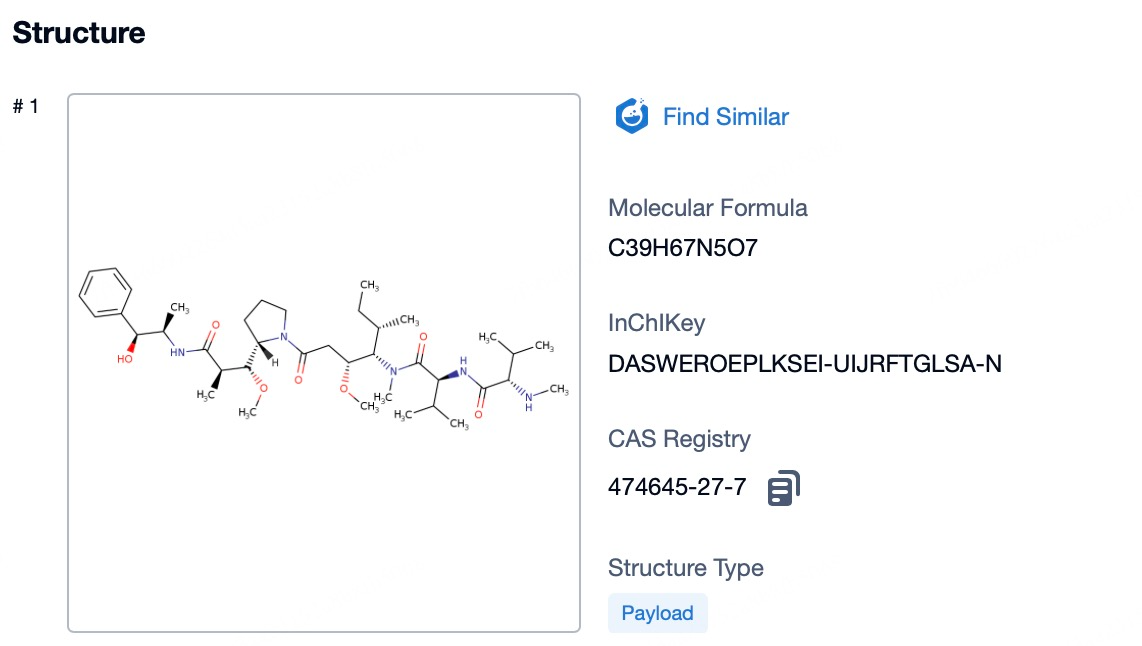
Patsnap Bio reveals that the cytotoxic payload in Enfortumab vedotin is monomethyl auristatin E (MMAE), a potent microtubule inhibitor and the primary anti-tumor component of the drug. MMAE effectively targets a variety of tumor cells by disrupting microtubule polymerization, thereby blocking cell division and proliferation, ultimately inducing tumor cell death. Upon the specific binding of the Enfortumab antibody to Nectin-4 on tumor cells, MMAE is precisely delivered into the tumor cells. Once internalized, MMAE inhibits microtubule polymerization, halting the cell division cycle and leading to apoptotic cell death, thus disrupting the tumor's proliferation cycle.
From Patsnap Bio, it is evident that Enfortumab vedotin consists of a fully humanized anti-Nectin-4 IgG1 monoclonal antibody conjugated with MMAE. This design significantly reduces the risk of immunogenicity. A fully humanized antibody means the sequence is almost entirely derived from human origin, minimizing the likelihood of triggering an immune response in patients. This feature allows the drug to be safely integrated with the patient’s immune system, reducing the risk of adverse reactions. The precise targeted delivery system enhances therapeutic efficacy while sparing normal cells, potentially lowering the occurrence of side effects.
Summary
The rapid growth of the ADC drug market confirms the development potential of this field. With the approval and market recognition of multiple ADC drugs such as Enhertu, Kadcyla, Trodelvy, and Padcev, the global ADC market size is expected to exceed $13 billion by 2024. The success of these drugs has not only brought new therapeutic hope to patients but also provided strong momentum for the biopharmaceutical industry, driving the development and market entry of more innovative drugs.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.
Refrence
- 1.Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther 7, 93 (2022). https://s10-doi-org.libproxy1.nus.edu.sg/s41392-022-00947-7
- 2.Heath, E. I. & Rosenberg, J. E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol 18, 93-103 (2021). https://s10-doi-org.libproxy1.nus.edu.sg/s41585-020-00394-5




