Chugai In-Licenses RNAi Therapeutic Zilebesiran for Hypertension with High Cardiovascular Risk
Chugai Pharmaceutical Co., Ltd. disclosed that it has secured a licensing deal with Roche concerning zilebesiran, an RNAi therapeutic under investigation for managing hypertension, originally developed by Alnylam Pharmaceuticals, Inc. This compound is presently being advanced by Roche in collaboration with Alnylam. As per the terms of the deal, Chugai will acquire the rights to commercialize zilebesiran within Japan. In exchange, Roche will be compensated with an initial payment and additional financial milestones.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.

High blood pressure is a critical condition that can trigger and exacerbate various cerebrovascular and cardiovascular incidents, such as strokes and heart attacks. Despite this, about 80% of individuals with hypertension experience persistently high blood pressure, indicating a substantial gap in effective treatment options. "In collaboration with Roche and Alnylam, Chugai is committed to advancing new treatment solutions promptly for those suffering from hypertension," stated Dr. Osamu Okuda, President and CEO of Chugai.
Zilebesiran, an investigational RNAi therapy, targets angiotensinogen (AGT) and acts by blocking its production in the liver, which may reduce levels of the vasoconstrictor angiotensin II. Alnylam has completed the Phase II KARDIA-2 global clinical trial, testing zilebesiran in individuals with mild to moderate, unmanaged hypertension. This study assessed the safety and effectiveness of a single subcutaneous injection of zilebesiran administered alongside one of three commonly used antihypertensive medications.
Zilebesiran is currently in development as a subcutaneous RNAi therapy aimed at those with hypertension who have not seen success with other treatments. AGT, an essential component in the Renin-Angiotensin-Aldosterone System, plays a crucial part in managing blood pressure, and blocking its action is known to reduce hypertension effectively. Through inhibiting AGT synthesis in the liver, zilebesiran might lead to sustained decreases in AGT levels and ultimately, lower angiotensin II levels. Utilizing Alnylam’s Enhanced Stabilization Chemistry Plus GalNAc-conjugate technology, zilebesiran is designed for less frequent dosing while maintaining selective and prolonged control of blood pressure. The results show that a single administration can sustain blood pressure reduction over a 24-hour period for up to six months.
In 2023, Roche and Alnylam formed a global collaboration to further develop and market zilebesiran. Under this partnership, both companies will jointly market the drug in the U.S., while Roche will exclusively handle its commercialization outside the U.S. Additionally, Chugai has secured commercialization rights in Japan through a licensing agreement with Roche. It is important to note that the safety and effectiveness of zilebesiran have yet to be approved by the FDA, PMDA, or any other regulatory body.
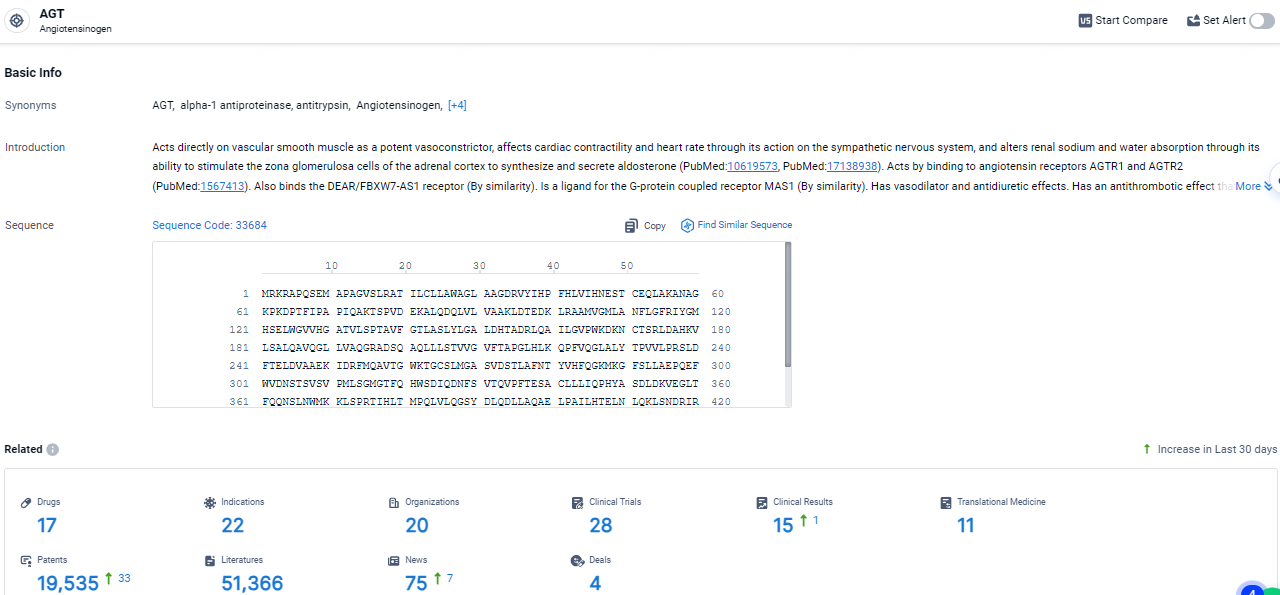
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 22, 2024, there are 17 investigational drugs for the AGT target, including 22 indications, 20 R&D institutions involved, with related clinical trials reaching 28, and as many as 19535 patents.
Zilebesiran targets AGT and is being investigated for its potential therapeutic applications in cardiovascular diseases, nervous system diseases, and urogenital diseases. The drug is currently in Phase 2 of development and shows promise in treating hypertension, Alzheimer's disease, and pre-eclampsia. Further research and clinical trials will be necessary to determine the drug's ultimate efficacy and safety profile.