TEPKINLY® (Epcoritamab) Approved by European Commission for Treatment of Adult Relapsed/Refractory Follicular Lymphoma
Genmab A/S (Nasdaq: GMAB) announced that the European Commission (EC) has given conditional marketing approval for TEPKINLY® (epcoritamab) for use as a monotherapy in adult patients with relapsed or refractory (R/R) follicular lymphoma (FL) following at least two prior systemic treatments. TEPKINLY is the first subcutaneous T-cell engaging bispecific antibody authorized for treating this specific patient group in the European Union (EU), European Economic Area (EEA) countries (Iceland, Liechtenstein, Norway), and Northern Ireland.
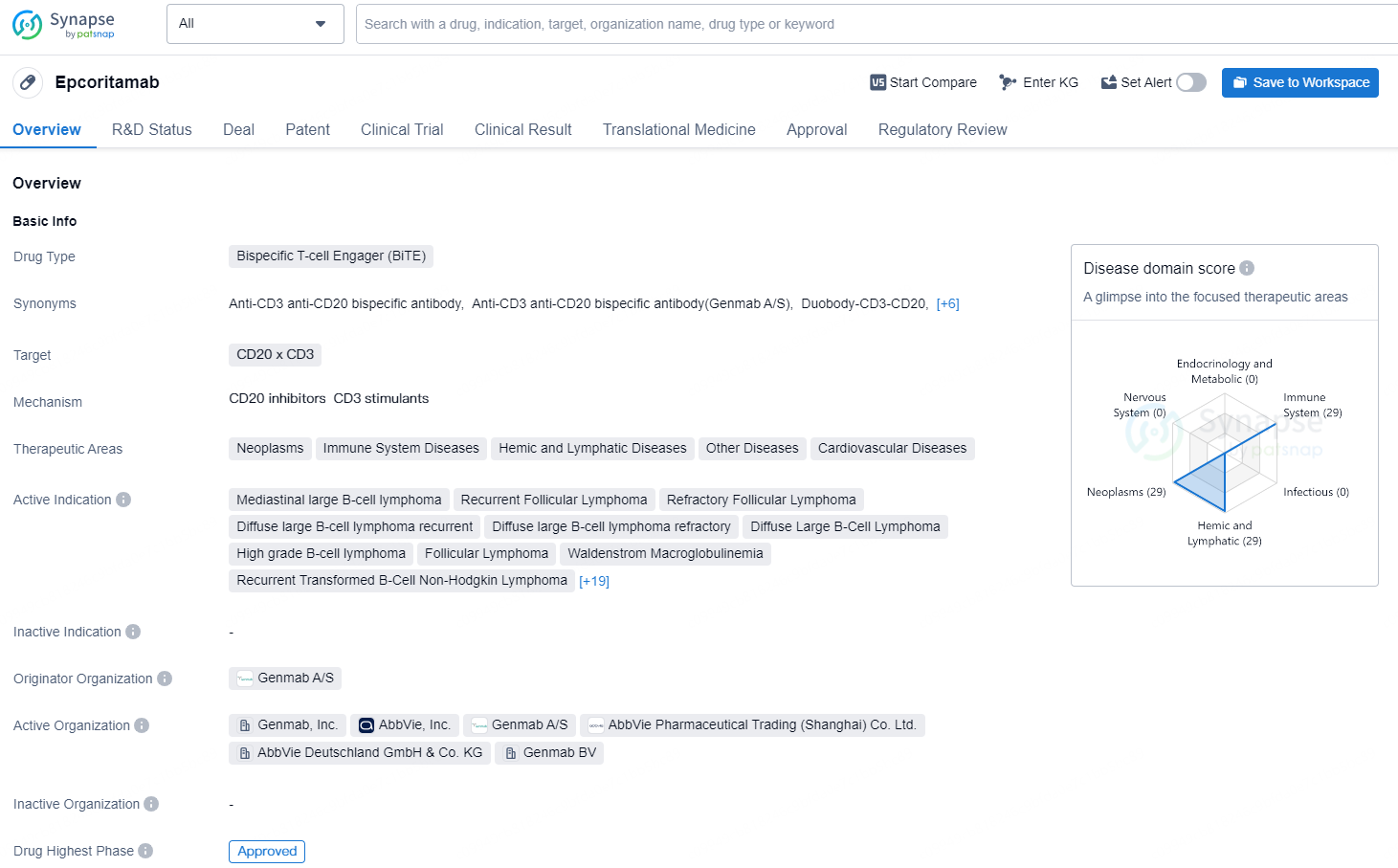
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 "Follicular lymphoma presents significant treatment challenges, and the recent approval of TEPKINLY for relapsed/refractory follicular lymphoma after at least two prior systemic therapies represents a significant advancement for patients in the European Union seeking more effective and safer treatment options," stated Jan van de Winkel, Ph.D., President and CEO of Genmab. "Together with our partner AbbVie, we remain dedicated to furthering the development of epcoritamab as a key therapy across various B-cell malignancies."
"Follicular lymphoma presents significant treatment challenges, and the recent approval of TEPKINLY for relapsed/refractory follicular lymphoma after at least two prior systemic therapies represents a significant advancement for patients in the European Union seeking more effective and safer treatment options," stated Jan van de Winkel, Ph.D., President and CEO of Genmab. "Together with our partner AbbVie, we remain dedicated to furthering the development of epcoritamab as a key therapy across various B-cell malignancies."
Follicular lymphoma (FL) is a generally indolent subtype of non-Hodgkin’s lymphoma (NHL) originating from B-cell lymphocytes. It is the second most prevalent form of NHL, accounting for 20-30% of all NHL cases, and makes up 10-20% of all lymphomas in Western countries. FL is deemed incurable, with no standardized treatment for third-line or later stages. Patients who do achieve remission frequently face relapse.
The conditional marketing authorization for TEPKINLY is based on data from the Phase 1/2 EPCORE® NHL-1 trial, which was an open-label, multi-cohort, multicenter, single-arm study examining TEPKINLY as a monotherapy in patients with R/R FL following two or more previous systemic treatments. The trial enrolled patients who were refractory to anti-CD20 monoclonal antibody therapy and an alkylating agent, with 70% having double refractory disease, 82% being refractory to their last prior treatment, and 52% experiencing disease progression within two years of starting their first systemic therapy. Published results in Lancet Haematology indicated that out of 128 patients treated with TEPKINLY, 83% had an overall response rate (ORR), and 63% achieved complete response (CR). At a median follow-up of 16.2 months, the median duration of response was 21.4 months, while the duration of complete response had not been reached.
The trial also included a separate planned optimization cohort evaluating 86 patients using a recommended 3-step-up dosing regimen to mitigate cytokine release syndrome (CRS), with no mandatory hospitalization during the first cycle. Under the optimized regimen, 40% of patients experienced Grade 1 CRS and 9% experienced Grade 2 CRS, with no Grade 3 or higher CRS reported. Additionally, no instances of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed in this cohort.
Epcoritamab's safety profile in the pivotal cohort was consistent with earlier reports from the EPCORE NHL-1 diffuse large B-cell lymphoma (DLBCL) cohort. In the combined safety population (n=382), the most frequent adverse reactions (≥ 20%) with TEPKINLY included CRS, injection site reactions, fatigue, viral infections, neutropenia, musculoskeletal pain, fever, and diarrhea. The most common severe adverse reaction (≥ 10%) was CRS, occurring in 34%. Fatal adverse reactions were observed in 3.7% of patients, primarily due to pneumonia (2.4%), viral infections (1.0%), and a single case of ICANS (0.3%).
"The European Commission's approval of epcoritamab is encouraging news for those affected by lymphoma," commented Kate Rogers, CEO of the Follicular Lymphoma Foundation. "Because relapsed or refractory follicular lymphoma poses significant treatment hurdles, particularly in later treatment stages, it is essential for patients and healthcare providers to have access to additional therapeutic options for this cancer."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
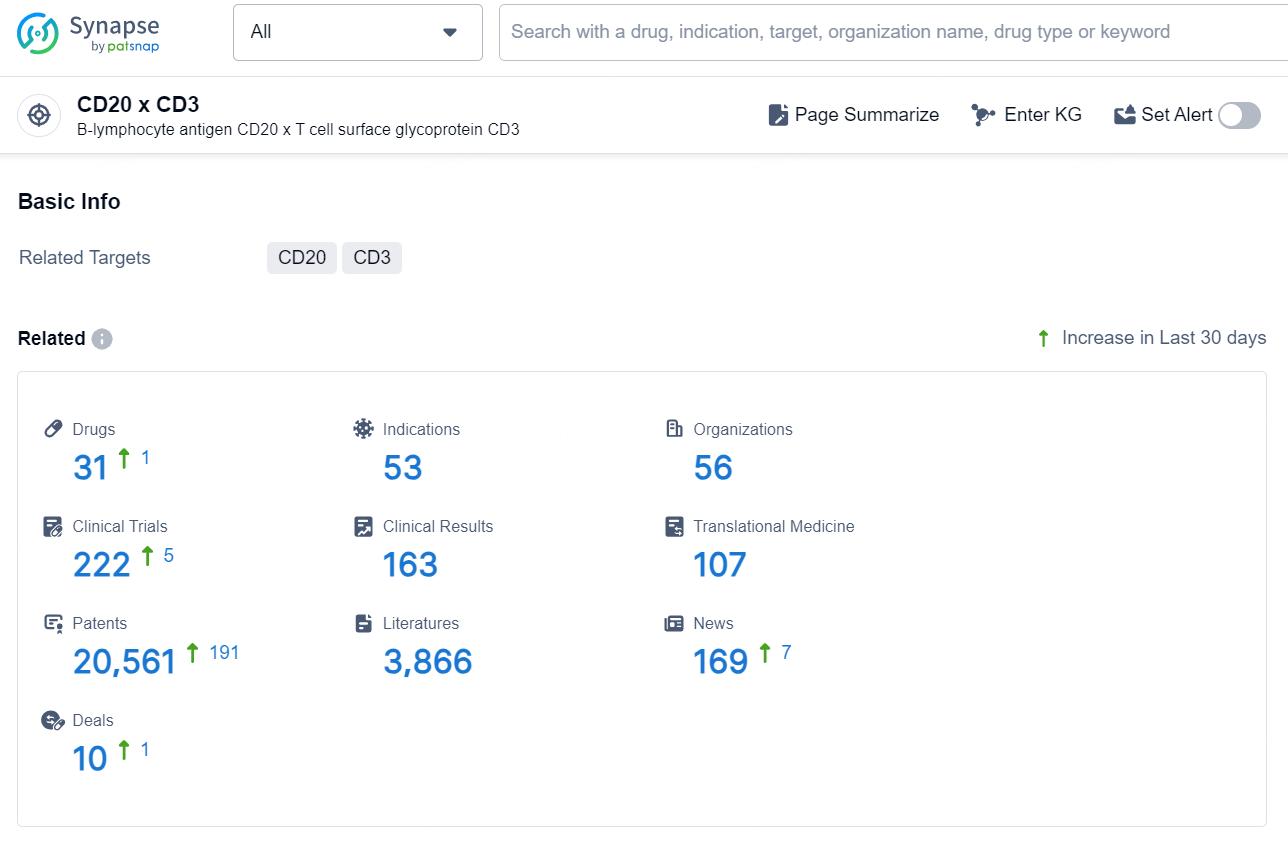
According to the data provided by the Synapse Database, As of August 22, 2024, there are 31 investigational drugs for the CD20 x CD3 targets, including 53 indications, 56 R&D institutions involved, with related clinical trials reaching 222, and as many as 20561 patents.
Epcoritamab is an IgG1-bispecific antibody created using Genmab’s proprietary DuoBody® technology and administered subcutaneously. Genmab’s DuoBody-CD3 technology is designed to direct cytotoxic T cells selectively to elicit an immune response toward target cell types. Epcoritamab is designed to simultaneously bind to CD3 on T cells and CD20 on B cells and induces T-cell-mediated killing of CD20+ cells.