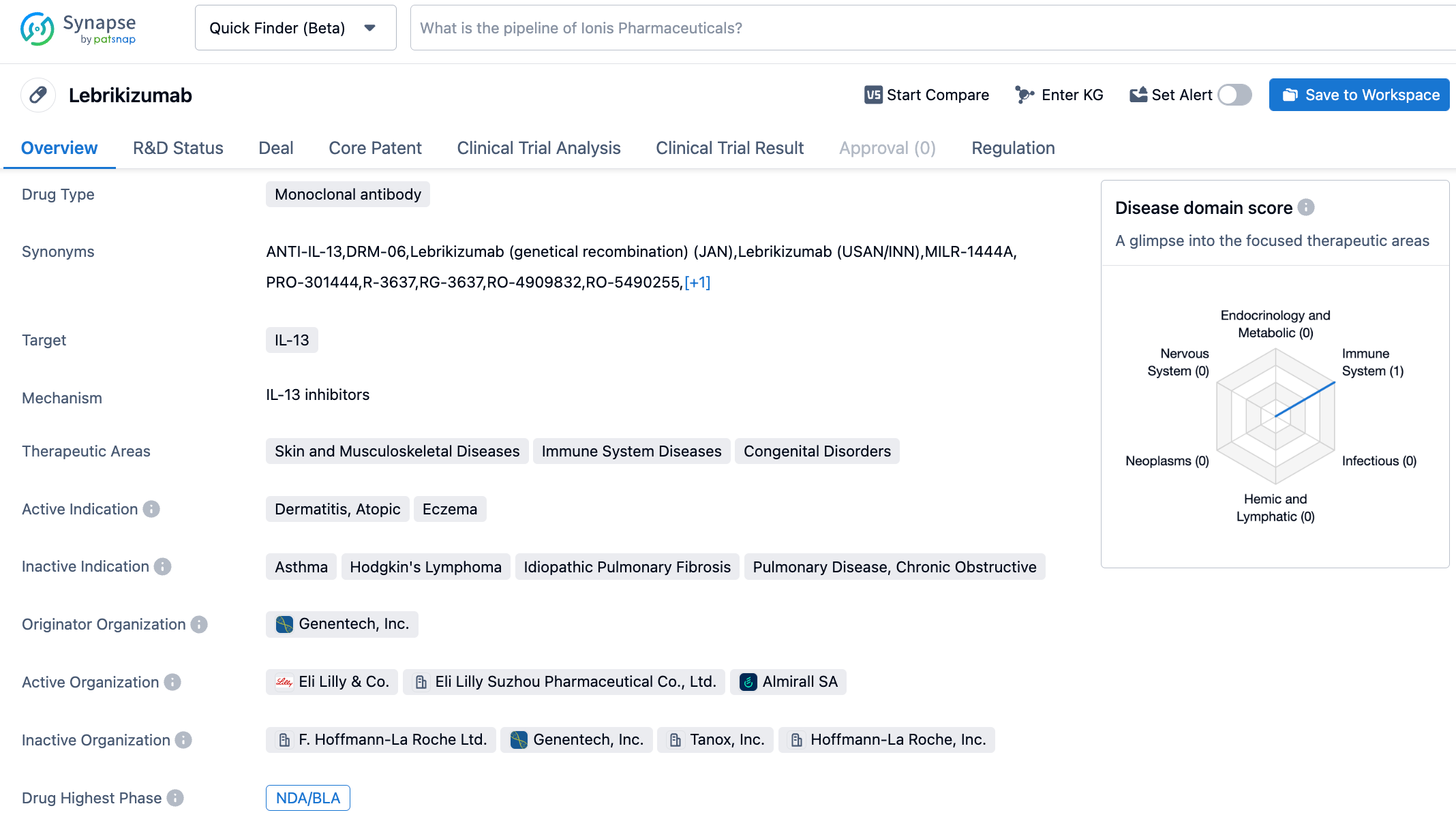
The Eli Lilly IL-13 inhibitor, lebrikizumab, demonstrates long-term efficacy for the treatment of moderate-to-severe atopic dermatitis
Recently, Eli Lilly announced the long-term therapeutic effects of its investigational IL-13 inhibitor, lebrikizumab, in patients with moderate to severe atopic dermatitis. The results indicated that patients with moderate to severe atopic dermatitis maintained clinical benefits such as clearing of skin symptoms, alleviation of itch, and reduction in disease severity over a two-year extension study with monthly administration of lebrikizumab.
Lebrikizumab is a novel anti-IL-13 monoclonal antibody with high bioavailability and a long half-life. It can bind to soluble IL-13 with high affinity and block IL-13 mediated signaling pathways. Previously, Lebrikizumab had received Fast Track designation from the FDA. Lebrikizumab was originally developed by Tanox under the name TNX-650; In 2007, Genentech acquired Tanox, making Lebrikizumab part of Roche's pipeline. Subsequently, in 2017 Dermira paid Roche an upfront payment of $80 million and a total milestone amount of $1.4 billion to acquire the global rights to Lebrikizumab. In June 2019, the European rights to the drug were sold to Almirall. In January 2020, Eli Lilly acquired Dermira for a total of $1.1 billion, with the global rights to Lebrikizumab (excluding Europe) falling into Eli Lilly's hands.
ADjoin is a two-year extension study. Patients were from the monotherapy trials ADvocate 1 and ADvocate 2 and from the lebrikizumab and topical corticosteroid combination trial Adhere. These patients had an Investigator's Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) or a reduction of 75% (EASI-75) from baseline in the average eczema area and severity index after 16 weeks of lebrikizumab treatment and were included in the ADjoin trial. Patients participating in the long-term extension trial needed to receive 250mg of lebrikizumab every two weeks or monthly. In the ADjoin trial, administering lebrikizumab either monthly or every two weeks for two years provided patients with lasting relief from skin symptoms and itch.
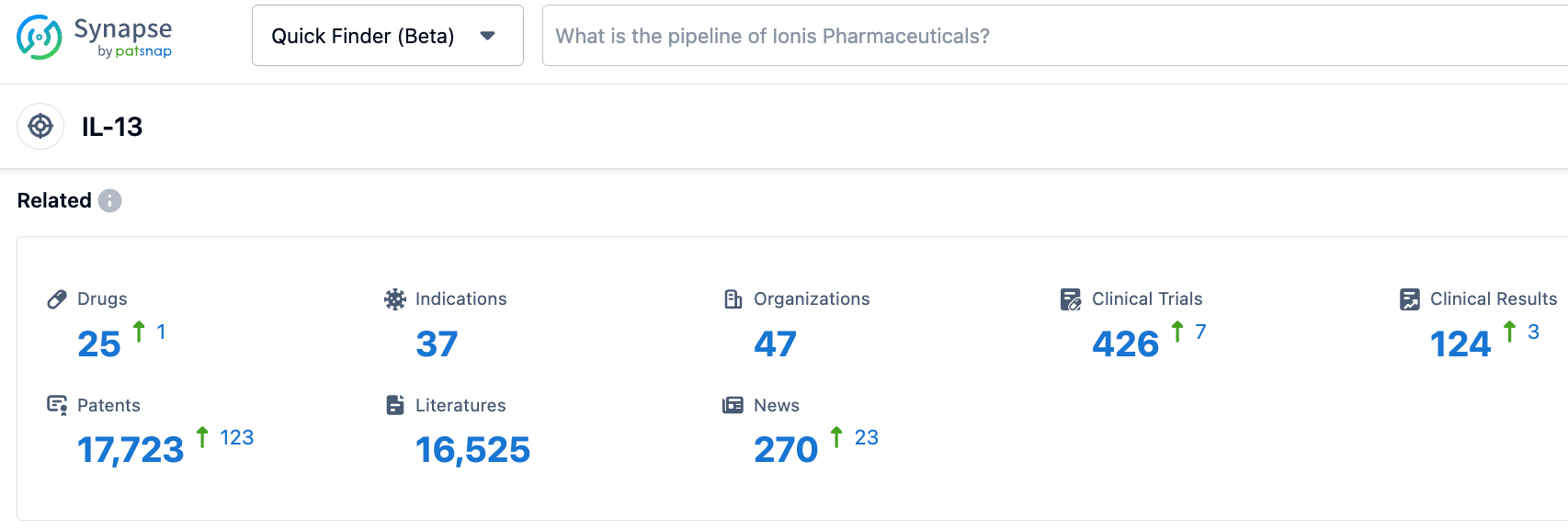
According to information disclosed by the Synapse database, as of October 31,2023, there were 25 investigational drugs targeting IL-13, covering 37 indications, with 47 research institutions involved, and there were 426 related clinical trials and as many as 17,723 patents...On October 2 of this year, the FDA announced that it had refused to approve Eli Lilly's Biologics License Application (BLA) for lebrikizumab for the treatment of moderate to severe atopic dermatitis (eczema). The main reason was that issues were discovered with a third-party Contract Manufacturing Organization (CMO) during inspection. There was no mention in the FDA's CRL of concerns about lebrikizumab's clinical data, safety, or labeling, so if the CMO issues can be effectively resolved, or if production is transferred to a new partner, the waiting time for approval may not be long. We look forward to the early approval of Lebrikizumab.