The FDA has cleared Mersana Therapeutics to resume the Phase 1 clinical trial for XMT-2056, the company announced
The U.S. Food and Drug Administration (FDA) has given permission to resume the Phase 1 clinical trial of XMT-2056, according to Mersana Therapeutics, Inc., a biopharmaceutical firm in the clinical stage. The company is committed to the discovery and development of a range of antibody-drug conjugates, addressing cancer types in sectors where there is a significant lack of medical solutions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
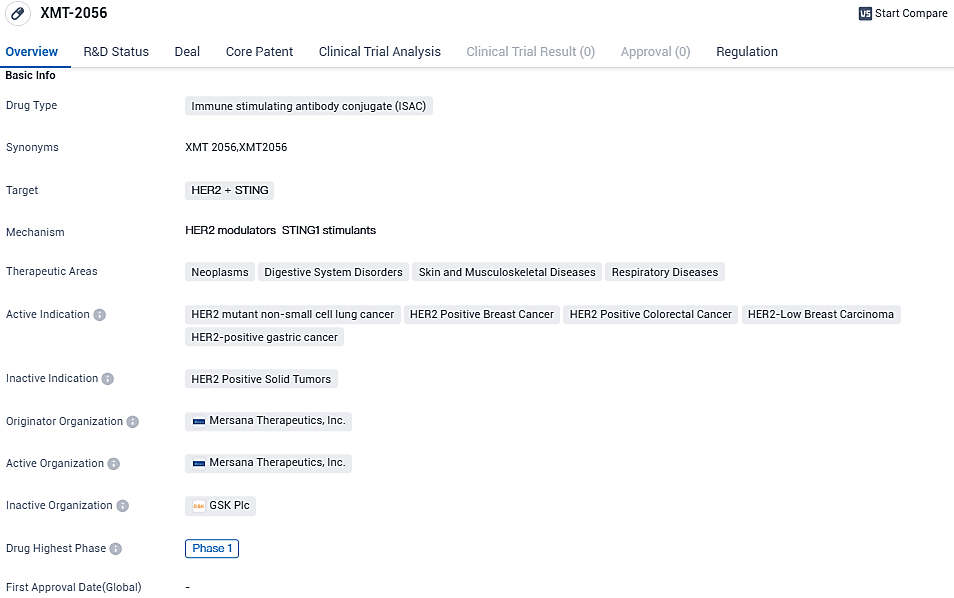
The Immunosynthen STING-agonist ADC known as XMT-2056 is a systemically administered treatment designed to target a new human epidermal growth factor receptor 2 (HER2) epitope. It then activates STING signaling locally within tumor cells and tumor-resident immune cells. This provides the potential for treatment of patients with high or low HER2 tumors independently, and in combination with standard care treatments.
Martin Huber, M.D., President and CEO of Mersana Therapeutics, stated "Our Phase 1 trial provided in-depth clinical data, including cytokine, pharmacokinetic, indicating that XMT-2056 holds great potency as an innate immune agonist."
"Focusing on patient safety, we have reduced the introductory dosage in our Phase 1 dose escalation plan. With the FDA's agreement on our path ahead, we are thrilled to have the chance to continue exploring the potential of XMT-2056 and our Immunosynthen ADC platform within the clinical arena," added Huber.
The Phase 1 open-label trial conducted across multiple centers is researching XMT-2056 on patients previously treated for severe/recurrent solid tumors expressing HER2 like breast, gastric, colorectal and non-small-cell lung cancers. The trial includes dosage escalation and dosage expansion phases, which will assess and profile the safety, tolerability and exposure of XMT-2056. Also, it will gauge the initial anti-tumor activity of the candidate, measured through the overall response rate, response duration, and disease control rate.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
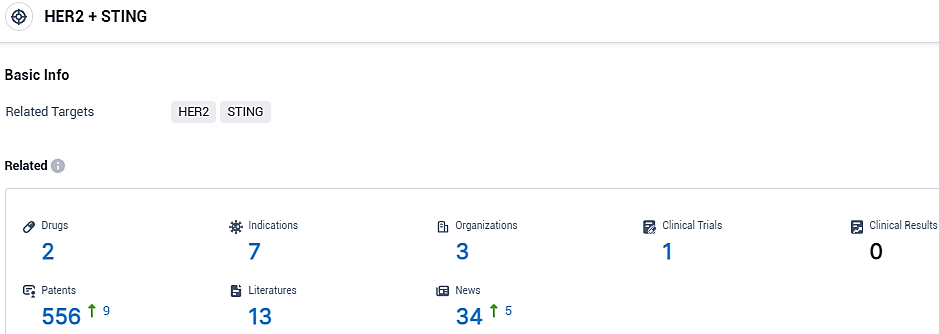
According to the data provided by the Synapse Database, As of November 6, 2023, there are 1 investigational drugs for the HER2 and STING target, including 3 indications, 1 R&D institutions involved, with related clinical trials reaching 3, and as many as 540 patents.
XMT-2056 is an immune stimulating antibody conjugate drug and has active indications in various types of cancers. The FDA has granted orphan drug designation to XMT-2056 for the treatment of gastric cancer. In August 2022, Mersana entered into a global collaboration providing GSK plc with an exclusive option to co-develop and commercialize XMT-2056. GSK has not exercised this option to date.