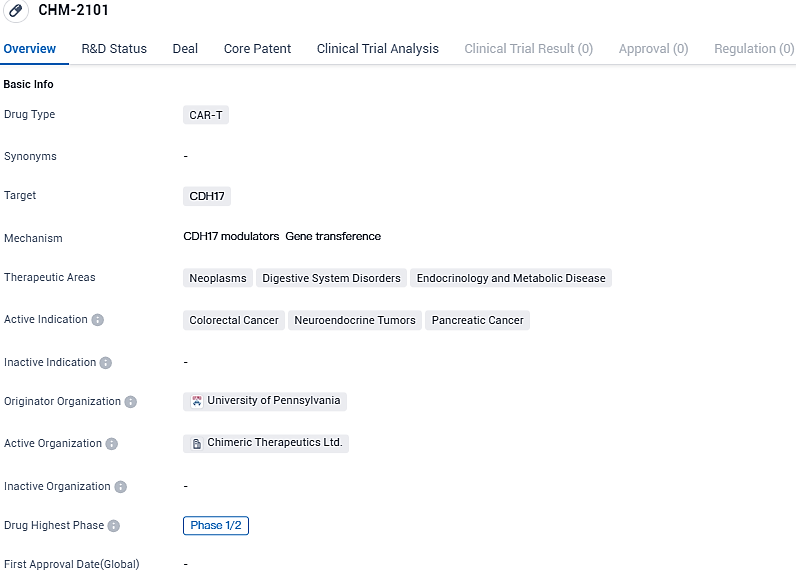
Chimeric Therapeutics reports FDA's approval of IND application for CHM 2101, a new CDH17 CAR T cell treatment targeting advanced GI cancer
Australian cell therapy pioneer, Chimeric Therapeutics, has disclosed that the US FDA has given the green light to the IND submission for CHM 2101. This novel CDH17 CAR T cell therapy created by Chimeric is initially planned for usage in treating gastrointestinal cancers.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The firm intends to carry out a broad-based, openly labelled Phase 1A/B clinical trial into CHM 2101 for subjects with advanced stages of Colorectal Cancer, Gastric Cancer and Neuroendocrine Tumours. CHM 2101 is a third-generation, innovative CDH17 CAR T cell treatment developed to target CDH17.
This is a cancer target linked with a dismal prognosis and metastasis in the most frequently occurring gastrointestinal tumours encompassing Colorectal Cancer, Gastric Cancer and Neuroendocrine Tumours. The CHM 2101 clinical program builds on preclinical studies conducted by leading immunotherapy scientist Xianxin Hua, MD, PhD, and his team at the Abramson Family Cancer Research Institute at the University of Pennsylvania, with results published in the leading scientific journal, Nature Cancer in March 2022.
These studies have shown that CHM 2101 has the ability to eliminate established tumours in seven types of cancer, without causing toxic damage to healthy tissues. Dr. Xianxin Hua, noted that the move from discovery of the CDH17 target and CAR T therapy in preclinical trials to initiating clinical trials in patients with GI-cancers and neuroendocrine tumours is exhilarating.
The professor of Cancer Biology in Penn's Perelman School of Medicine stated, "This step is key in the formation of a completely new CAR T treatment for GI-cancers and neuroendocrine tumours, and offers new possibilities for patients who have become unresponsive to existing treatments." Upon the receipt of FDA IND clearance, Chimeric is set to launch a Phase 1/2 multi-location clinical trial for patients with advanced stages of Colorectal Cancer, Gastric Cancer and Neuroendocrine Tumours. It is anticipated that the trial will commence patient recruitment in 2024.
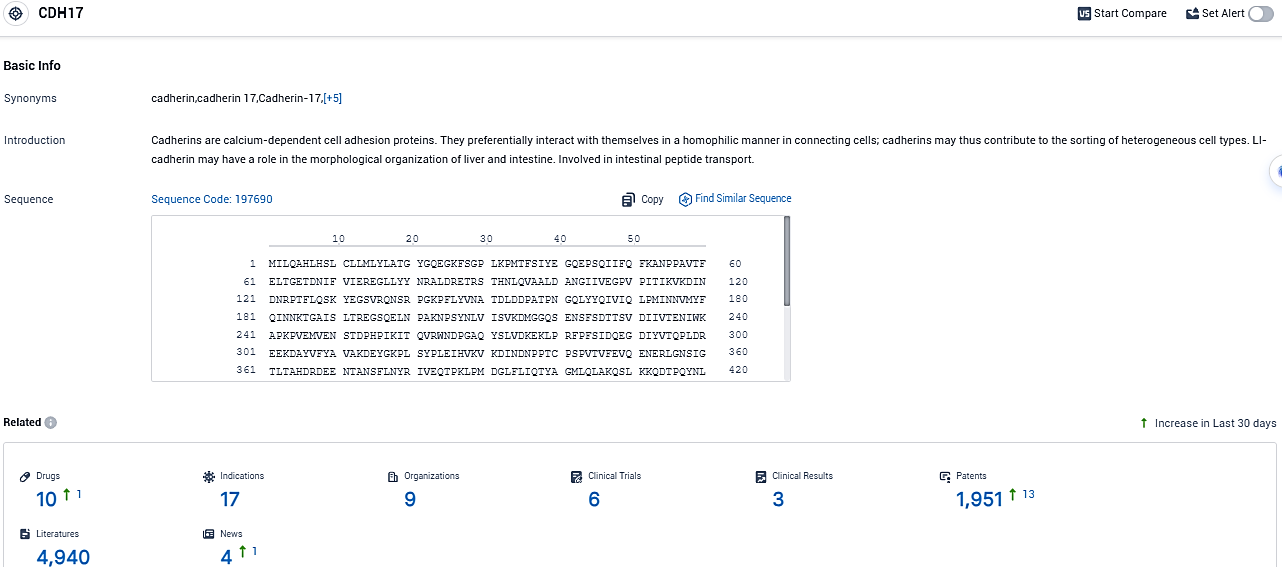
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 6, 2023, there are 10 investigational drugs for the CDH17 target, including 17 indications, 9 R&D institutions involved, with related clinical trials reaching 6, and as many as 4940 patents.
CHM 2101, a pioneering 3rd generation CDH17 CAR T, was birthed from the globally acclaimed cell therapy institution, the University of Pennsylvania. The initial indications of its potential were revealed in Nature Cancer in March 2022, showcasing complete removal of tumors in seven distinct cancer types. Presently, CHM 2101 is under preclinical investigation, and there are preparations underway for initiating a phase 1A clinical test on gastrointestinal and neuroendocrine tumours.