The primary endpoint achieved by FASENRA in the Phase III MANDARA trial for eosinophilic granulomatosis with polyangiitis
Positive high-level results from AstraZeneca's MANDARA Phase III study revealed that FASENRA(benralizumab) achieved the trial's primary goal and presented comparable remission rates to mepolizumab in individuals with eosinophilic granulomatosis with polyangiitis who were undergoing treatment with oral corticosteroids, with or without consistent immunosuppressive therapy.
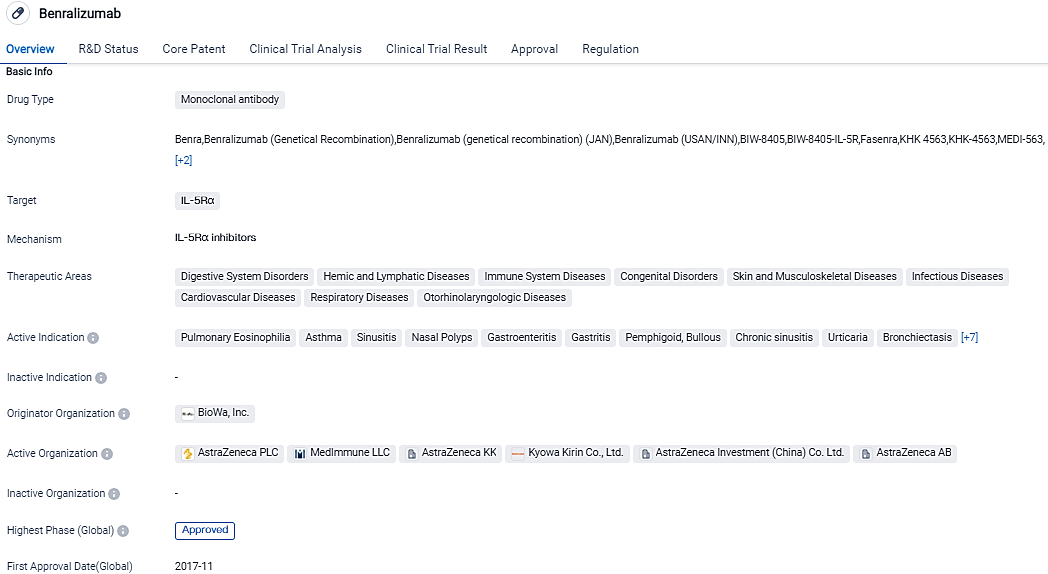
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The MANDARA study, the first Phase III biologics trial to pit two treatments head-to-head trial of EGPA, compared FASENRA's efficacy and safety against mepolizumab, which is currently the only endorsed treatment. In the trial that was conducted in a double-blind manner, patients were randomly assigned to receive either a singular 30mg subcutaneous shot of FASENRA or three 100mg subcutaneous doses of mepolizumab every month.
EGPA, a rare disease triggering inflammation in small to middle-sized blood vessels due to autoimmune reactions, affects about 50% of its sufferer population also has severe eosinophilic asthma that started in adulthood. If left untreated, the condition can progressively damage various organs including lungs, skin, heart, gastrointestinal system, and nerves, at times with life-threatening results.
“The encouraging results from the MANDARA trial provide optimism as treatment options are limited for those suffering from eosinophilic granulomatosis with polyangiitis," said trial Chief Investigator, Dr. Michael Wechsler. "The trial showcases the potential of a biologic medication administered through a single monthly injection to match current standard care remission rates, underscoring the potential of benralizumab as a treatment alternative for eosinophilic granulomatosis with polyangiitis.”
Sharon Barr, Executive Vice President at BioPharmaceuticals R&D, AstraZeneca, commented on the favorable MANDARA findings “FASENRA, which uniquely works by directly targeting eosinophils, could help patients achieve remission from the demoralizing impact of this inflammatory ailment with a simplified once-monthly subcutaneous injection."
FASENRA, a monoclonal antibody, latches onto the IL-5 receptor alpha found on eosinophils. This triggers natural killer cells, leading to an almost entire depletion of blood and tissue eosinophils in most patients through programmed cell death.
The 52-week Phase III MANDARA trial, featuring a randomized, double-blind, double-dummy, active-controlled, parallel-group, multi-center design, compared the effectiveness and safety of FASENRA to mepolizumab in adult patients with relapsing or refractory EGPA. In this concealed trial, 140 participants were randomly divided to receive either FASENRA or mepolizumab every four weeks, delivered through subcutaneous injections.
Patients completing the double-blind treatment stage across 52 weeks may qualify to proceed to an open-label extension phase, granting each patient an opportunity to receive at least one year of treatment with open-label FASENRA.
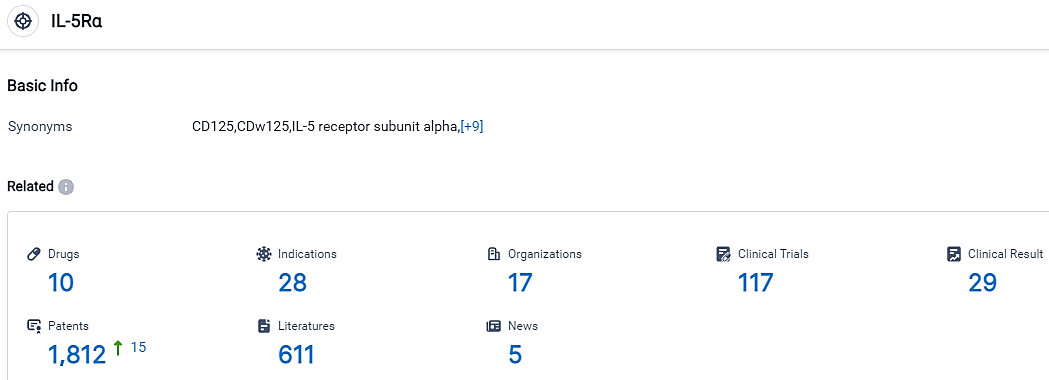
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 14, 2023, there are 10 investigational drugs for the IL-5Rα target, including 28 applicable indications,17 R&D institutions involved, with related clinical trials reaching 117,and as many as 1812 patents.
FASENRA is currently approved as an add-on maintenance treatment for SEA in the US, EU, Japan and other countries, and is approved for self-administration in the US, EU and other countries. The FDA granted Orphan Drug Designation for FASENRA for EGPA in 2018 and AstraZeneca continues to explore FASENRA’s potential beyond severe asthma, as a treatment across many diseases where eosinophils are expected to play a role.