"The Regulator" in Cancer Treatment: Interleukins
Interleukins (IL), also known as "white cell stimulators," are a type of cytokines first found to be produced by white blood cells and act between them. Later, it was discovered that interleukins can be produced by various cells and affect various cells. They serve as a communication tool between innate and adaptive immune cells, as well as non-immune cells and tissues. They play a crucial role in transmitting information, activating and regulating immune cells, mediating cell activation, proliferation and differentiation, and playing a significant role in inflammatory responses. Moreover, interleukins are closely linked to the occurrence and development of various kinds of cancer.
So far, at least 29 interleukins have been detected, named from IL1 to IL29, all possessing complex functions. Based on the homology of cytokine structures, interleukins can be divided into several protein families such as the IL-1 family, IL-2 family, IL-6 family, IL-10 family, chemokine family, and so forth. Each type of interleukin comes from different cells and acts on different target cells. The majority of interleukins participate in autoimmune disorders and inflammatory diseases, while some are associated with tumor formation.
In recent years, with the deepening of research, the therapeutic potential of interleukins has been considered a hot topic in the area of basic and translational cancer research. The interleukin family has emerged a large number of drug targets, many of which have been listed and even occupied a large market share in the treatment of similar diseases. An increasing number of clinical trials also highlight their value as therapeutic drugs and targets.
Interleukins Competitive Landscape
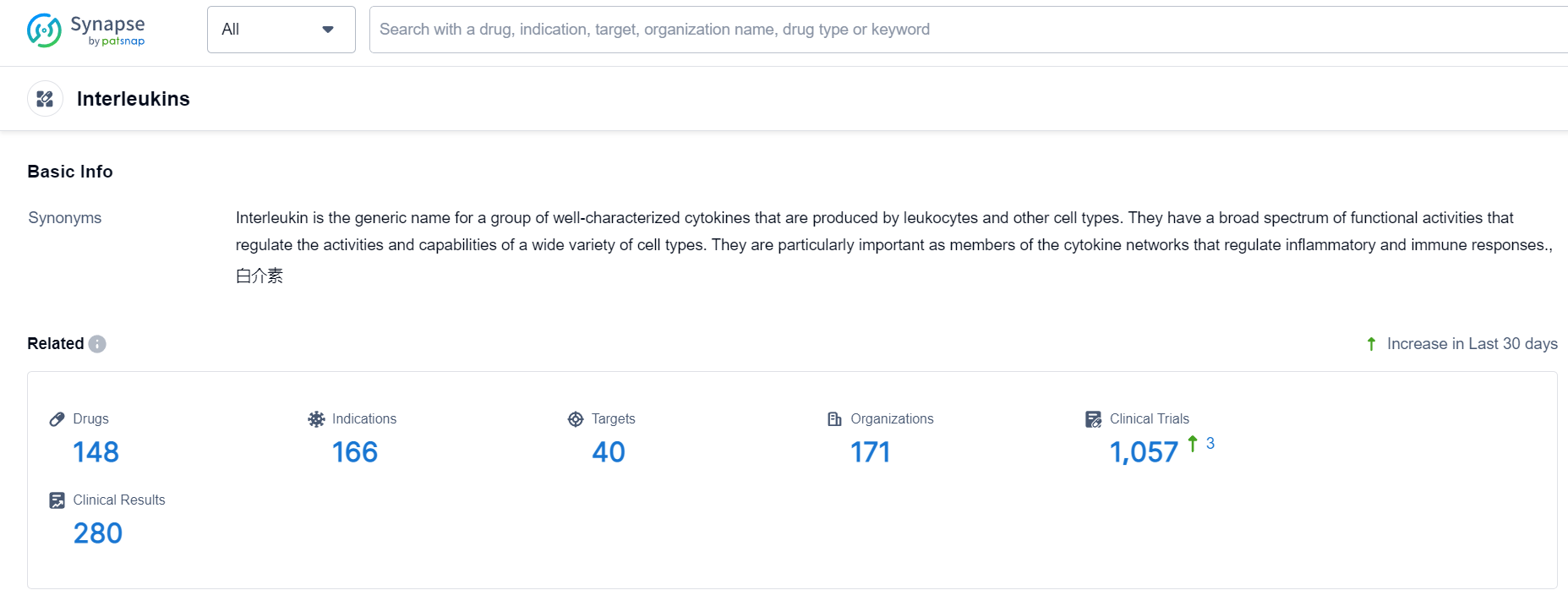
According to Patsnap Synapse, as of 24 Sep 2023, there are a total of 148 Interleukins drugs worldwide, from 171 organizations, covering 40 targets, 166 indications, and conducting 1057 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
Approved Interleukins Medicinal: Recombinant human interleukin-11
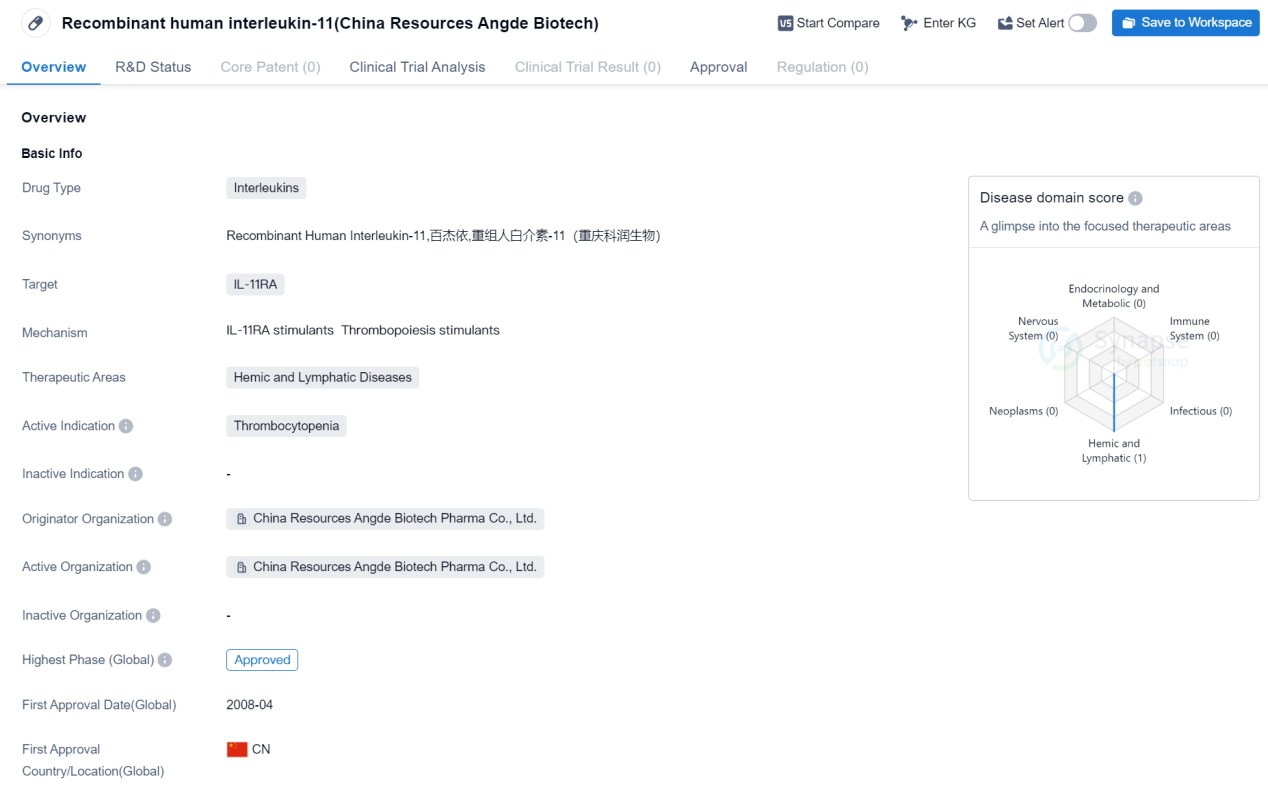
Recombinant human interleukin-11 (China Resources Angde Biotech) is a drug classified under the category of Interleukins. It specifically targets IL-11RA and is primarily used for the treatment of hemic and lymphatic diseases. The active indication for this drug is thrombocytopenia, a condition characterized by a low platelet count in the blood.
China Resources Angde Biotech Pharma Co., Ltd. is the originator organization behind this drug. The highest phase of development for this drug is approved.
Interleukins are a group of proteins that play a crucial role in regulating the immune system. By targeting IL-11RA, recombinant human interleukin-11 aims to address thrombocytopenia, a condition characterized by a low platelet count. Platelets are essential for blood clotting, and a deficiency in platelets can lead to excessive bleeding and other complications.
The approval of this drug in global market highlights its potential effectiveness in treating thrombocytopenia. It also signifies the successful development and regulatory processes undertaken by China Resources Angde Biotech Pharma Co., Ltd. to bring this drug to market.
In summary, recombinant human interleukin-11 (China Resources Angde Biotech) is an approved drug targeting IL-11RA for the treatment of thrombocytopenia. Developed by China Resources Angde Biotech Pharma Co., Ltd., it has obtained approvals in global markets. This drug represents a significant advancement in the field of biomedicine, specifically in the treatment of hemic and lymphatic diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Recombinant human interleukin-2 (rhIL-2)
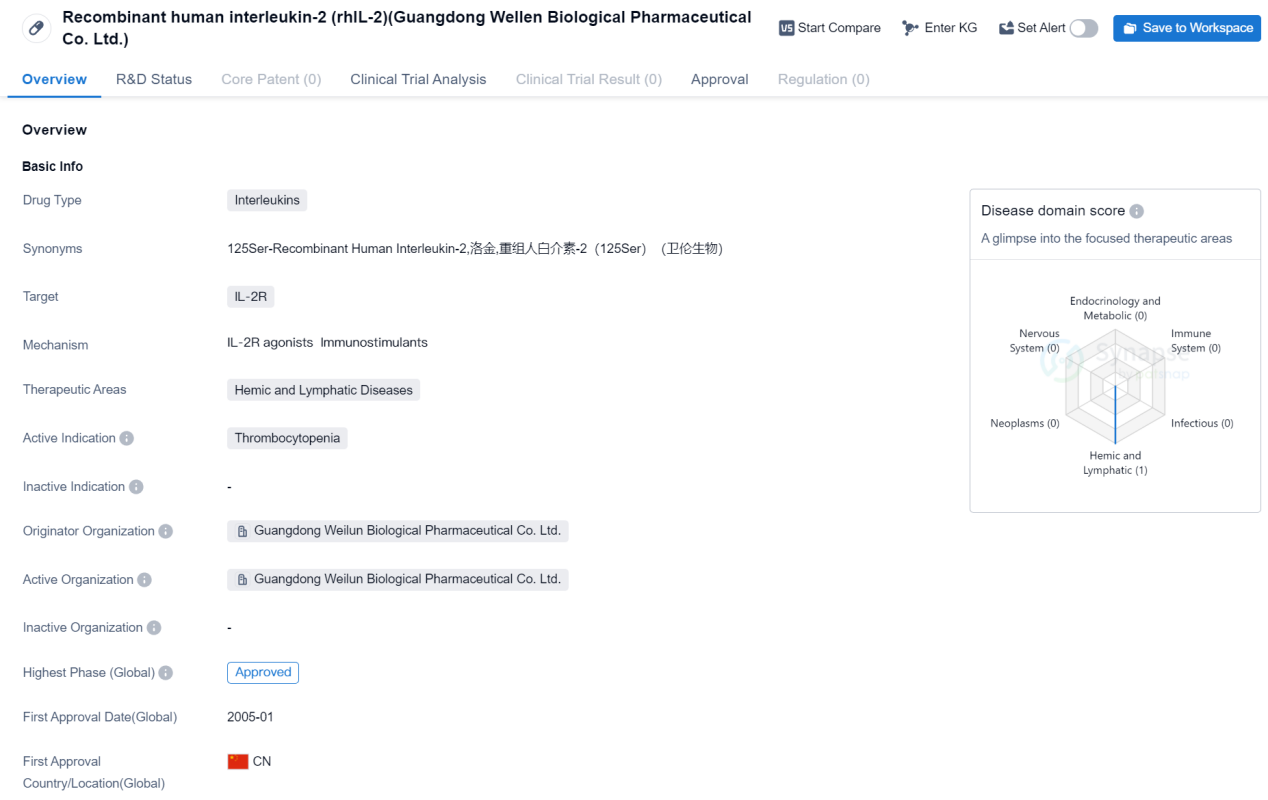
Recombinant human interleukin-2 (rhIL-2) is a drug developed by Guangdong Wellen Biological Pharmaceutical Co. Ltd. It falls under the category of Interleukins and targets the IL-2R. The drug is primarily used for the treatment of Thrombocytopenia, a condition characterized by a low platelet count.
Thrombocytopenia is a disorder that affects the hemic and lymphatic system, specifically the blood cells responsible for clotting. It can lead to excessive bleeding and bruising, making it a significant concern for patients. The approval of rhIL-2 provides a potential solution for individuals suffering from this condition.
Guangdong Wellen Biological Pharmaceutical Co. Ltd. is the originator organization behind the development of rhIL-2. The first approval for rhIL-2 was granted in China in January 2005, making it available to patients in that country.
As an expert in the pharmaceutical industry, it is important to note that rhIL-2's approval status in other countries is not provided in the given information. However, the fact that it has reached the highest phase of approval globally suggests that it has undergone rigorous testing and evaluation, meeting the necessary regulatory requirements.
Recombinant human interleukin-2 offers a promising treatment option for patients with Thrombocytopenia. By targeting the IL-2R, it aims to address the underlying causes of the condition and improve platelet count. The drug's approval in China signifies its potential to make a significant impact on the management of Thrombocytopenia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In conclusion, rhIL-2, developed by Guangdong Wellen Biological Pharmaceutical Co. Ltd., is an approved drug targeting IL-2R for the treatment of Thrombocytopenia. Its approval in China in 2005 indicates its potential to address the needs of patients suffering from this condition. Further information regarding its approval status in other countries would provide a more comprehensive understanding of its global availability.