TiumBio Reveals Promising Early Data for Hemophilia Candidate TU7710 at ISTH 2024
TiumBio Co., Ltd., a biopharmaceutical firm in the clinical stages of creating pioneering therapies for rare and untreatable conditions, revealed today that it shared clinical results of its hemophilia therapy, TU7710, at the 32nd Congress of the International Society on Thrombosis and Haemostasis. Additionally, the company is initiating new global collaborations.
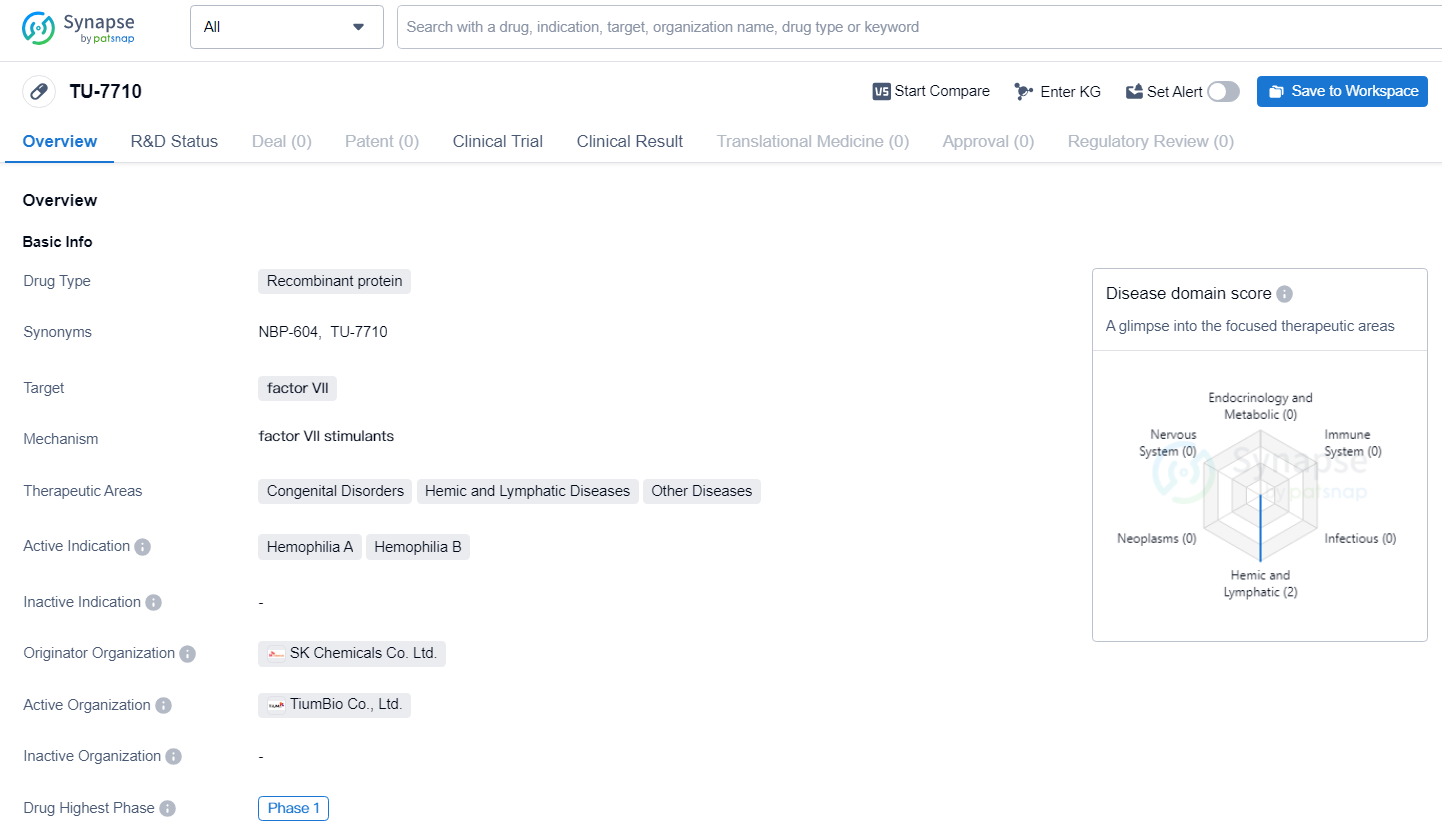
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
TiumBio is actively running a double-blind, placebo-controlled Phase 1a clinical study to evaluate the pharmacokinetics, pharmacodynamics, and safety profile of TU7710 in healthy male adults. During a recent conference, interim data from 32 participants were unveiled, covering dose ranges from 100 μg/kg to 800 μg/kg.
According to the clinical data shared at the ISTH conference, TU7710 exhibited an average half-life between 10.4 and 16.6 hours across different dosage groups. This is significantly longer—5 to 7 times—than the 2.3-hour half-life of NovoSeven, a standard hemophilia treatment used for patients with antibodies against conventional therapies. More importantly, no serious adverse or thromboembolic events were noted, and the majority of adverse events were mild in nature.
The term "half-life" describes the time required for the drug's concentration in the bloodstream to fall by half, which is crucial for the drug's efficacy duration. Given NovoSeven's brief half-life, hemophilia A or B patients with inhibitors are recommended to receive doses every two hours during bleeding episodes to achieve hemostasis, posing a challenge for both patients and healthcare providers.
TU7710 represents a recombinant factor VIIa with an extended half-life, achieved through TiumBio's transferrin fusion technology. The company aims to launch a Phase 1b clinical trial of TU7710 involving hemophilia patients in Europe by the latter half of this year.
“While NovoSeven has been a cornerstone in hemophilia treatment, generating $10 billion in sales over the last nine years, its short half-life presents notable medical challenges,” commented Hun-Taek Kim, Ph.D., MBA, CEO of TiumBio. “The prolonged half-life of TU7710, demonstrated in preclinical and initial human trials, significantly enhances the drug’s commercial potential,” he concluded..
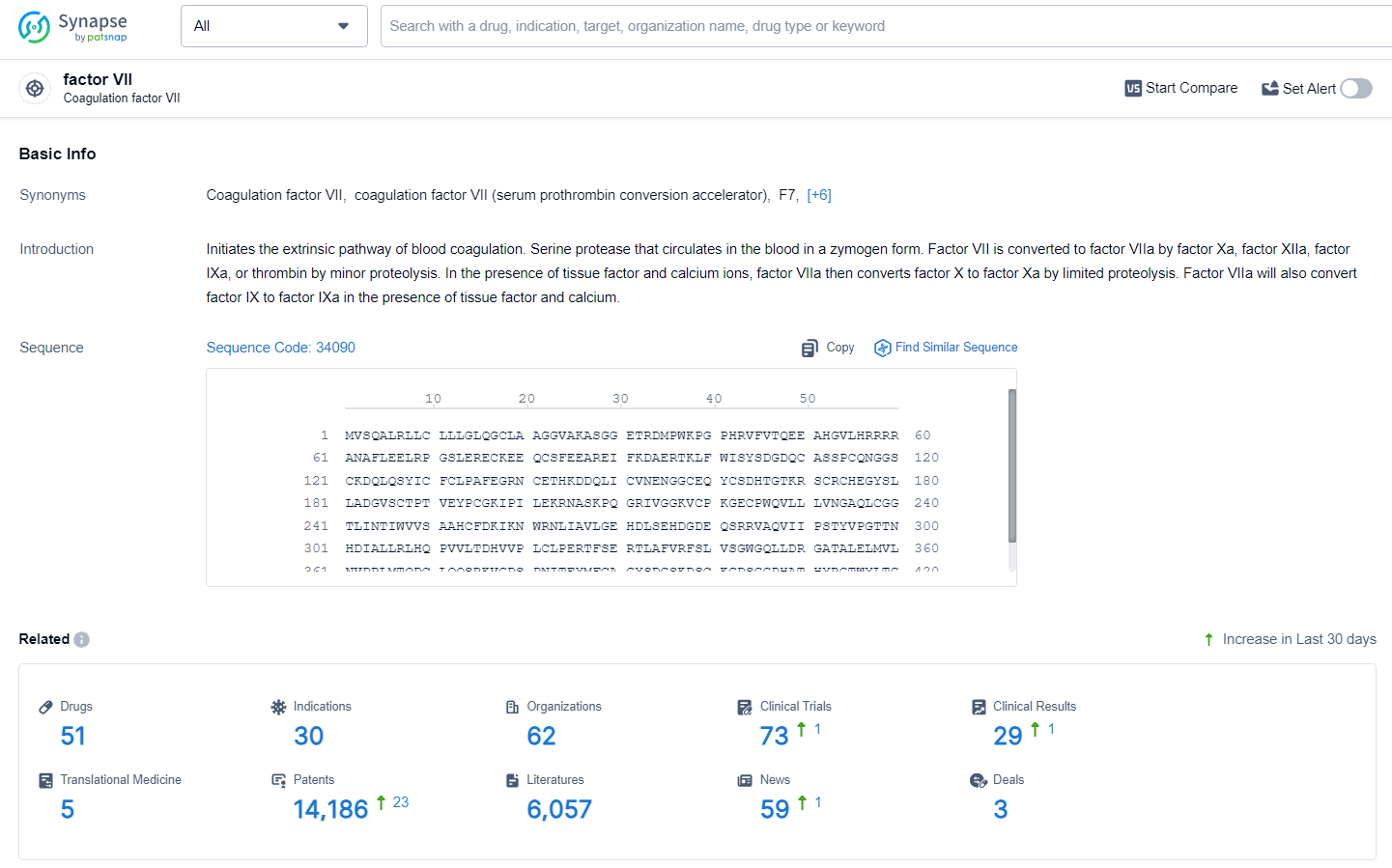
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 28, 2024, there are 51 investigational drugs for the factor VIIa target, including 30 indications, 62 R&D institutions involved, with related clinical trials reaching 73, and as many as 14186 patents.
TU-7710 holds promise for addressing the unmet medical needs of patients with hemophilia A and B, as well as other related hemic and lymphatic diseases. As the drug progresses through clinical development, further data and insights will be needed to assess its potential impact on patient outcomes and its eventual market availability.