Novavax COVID-19 Vaccine for 2024-2025 Approved and Advised for U.S. Use
Novavax, Inc. (Nasdaq: NVAX), a global entity focused on developing protein-based vaccines incorporating its Matrix-M™ adjuvant, announced that the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) has been granted Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) for active immunization to prevent COVID-19 in individuals aged 12 and up. The vaccine from Novavax is part of the recommendations put forth by the U.S. Centers for Disease Control and Prevention (CDC) on June 27, 2024.
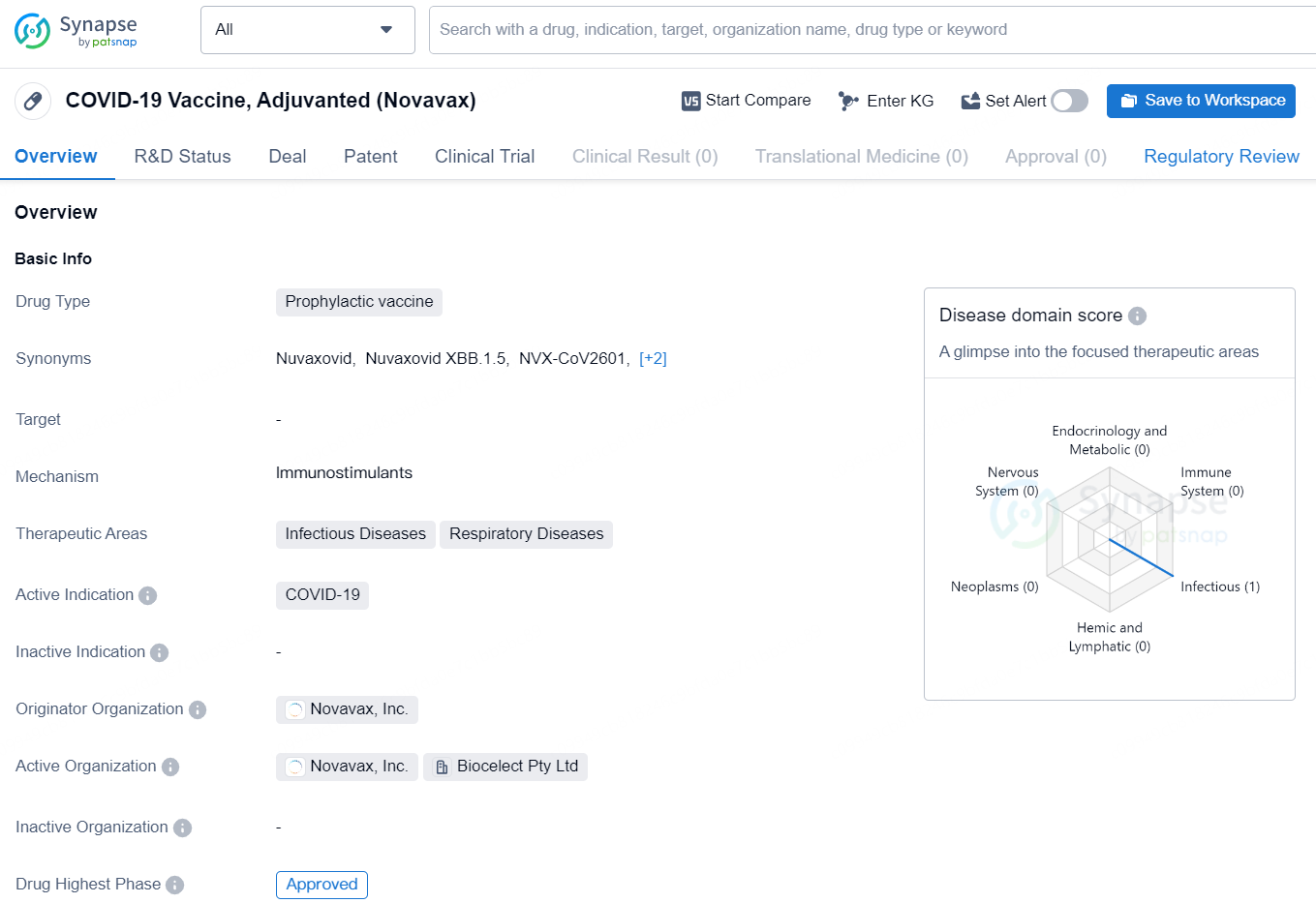
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Pre-filled syringes containing the vaccine will be available at numerous locations, including retail chains, independent pharmacies, and regional grocery stores, following the release of vaccine batches by the Center for Biologics Evaluation and Research.
"The authorization we received today allows Novavax to introduce our revised COVID-19 vaccine in the U.S. with pre-filled syringes. We have made significant efforts to make this accessible at thousands of locations across the country," stated John C. Jacobs, President and Chief Executive Officer of Novavax. "Our updated vaccine is designed to target JN.1, the 'parent strain' of the variants currently in circulation, and it has demonstrated strong cross-reactivity against various viruses within the JN.1 lineage, such as KP.2.3, KP.3, KP.3.1.1, and LB.1."
In June, the CDC's Advisory Committee on Immunization Practices unanimously endorsed a universal recommendation for using the 2024-2025 COVID-19 vaccines, which are either authorized under Emergency Use Authorization (EUA) or approved through a Biologics License Application, for individuals aged six months and older, without consideration of specific viral strains. At the June 2024 U.S. FDA Vaccines and Related Biological Products Advisory Committee meeting, it was discussed that there is a public health advantage in targeting JN.1, the parent strain of the most prevalent current variants. Novavax submitted its application for JN.1 in accordance with guidance from the U.S. FDA, European Medicines Agency (EMA), and the World Health Organization to address the JN.1 lineage this fall.
The EUA was granted based on non-clinical data indicating that Novavax’s updated vaccine offers protection and cross-reactivity against JN.1 and numerous JN.1 lineage viruses, including KP.2.3, KP.3, KP.3.1.1, and LB.1. Clinical trial data showed that common adverse reactions to Novavax’s original COVID-19 vaccine (NVX-CoV2373) included headache, nausea or vomiting, muscle pain, joint pain, tenderness at the injection site, injection site pain, fatigue, and malaise.
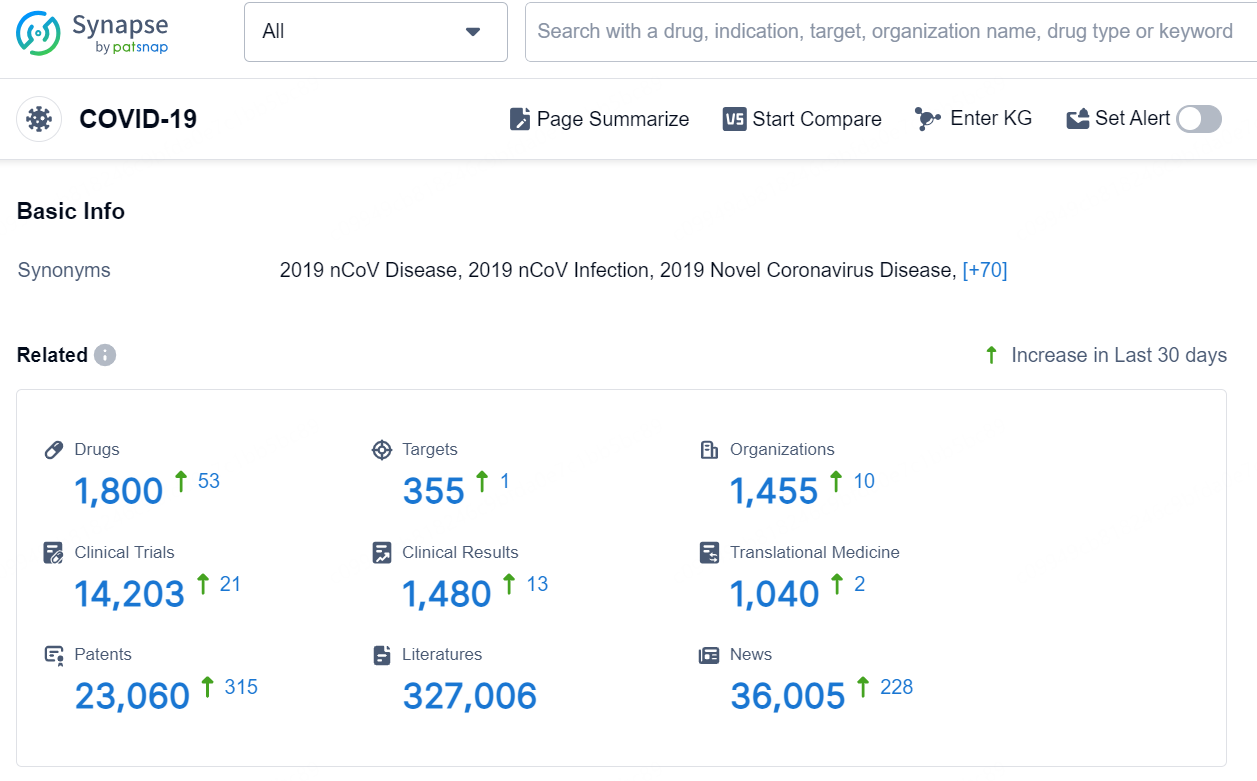
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 3, 2024, there are 1800 investigational drugs for the COVID-19, including 355 targets, 1455 R&D institutions involved, with related clinical trials reaching 14203, and as many as 23060 patents.
NVX-CoV2705 is an updated version of Novavax’s prototype COVID-19 vaccine (NVX-CoV2373) formulated to target the JN.1 variant. It is a protein-based vaccine made by creating copies of the surface spike protein of SARS-CoV-2 that causes COVID-19. With Novavax’s unique recombinant nanoparticle technology, the non-infectious spike protein serves as the antigen that primes the immune system to recognize the virus, while Novavax’s Matrix-M adjuvant enhances and broadens the immune response. The vaccine is packaged as a ready-to-use liquid formulation and is stored at 2° to 8°C, enabling the use of existing vaccine supply and cold chain channels.