Tourmaline Bio Starts Phase 2 Trials of TOUR006 for Cardiovascular Disease
Tourmaline Bio, Inc., a biotechnology firm in the advanced stages of clinical development focusing on breakthrough treatments for severe immune and inflammatory disorders, revealed that it has administered the first dose to a patient in its Phase 2 TRANQUILITY trial. This event signifies the official start of their clinical program for TOUR006, an extended-duration, fully human anti-IL-6 monoclonal antibody, aimed at treating atherosclerotic cardiovascular disease and other related cardiovascular conditions.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 TRANQUILITY represents a randomized, double-blind, placebo-controlled study targeting patients with an inflammatory risk profile, indicated by elevated baseline high-sensitivity C-reactive protein, and chronic kidney disease.
TRANQUILITY represents a randomized, double-blind, placebo-controlled study targeting patients with an inflammatory risk profile, indicated by elevated baseline high-sensitivity C-reactive protein, and chronic kidney disease.
"There's an urgent need for novel treatments for the vast number of patients who continue to face a high risk of heart attack, stroke, acute limb ischemia, and mortality, despite conventional risk factor adjustments," stated Emil deGoma, MD, Senior Vice President of Medical Research at Tourmaline and former Medical Director of the Preventive Cardiovascular Program at the University of Pennsylvania.
"Evidence converging from IL-6 human genetic research, epidemiological investigations, and mechanistic studies, along with data from clinical trials on IL-6 inhibition, strongly supports the therapeutic potential of IL-6 inhibition for patients with ASCVD as well as other cardiovascular conditions, including heart failure," deGoma added.
The TRANQUILITY study design incorporates insights from six previous Phase 1 and Phase 2 studies of TOUR006. If successful, outcomes from the Phase 2 TRANQUILITY study are expected to prepare Tourmaline for Phase 3 trials in 2025 for ASCVD and other cardiovascular conditions.
"TOUR006 presents a unique 'pipelines in a product' opportunity, addressing both severe cardiovascular diseases driven by IL-6 inflammation and serious autoimmune disorders, such as thyroid eye disease," elaborated Sandeep Kulkarni, MD, Co-Founder and Chief Executive Officer of Tourmaline.
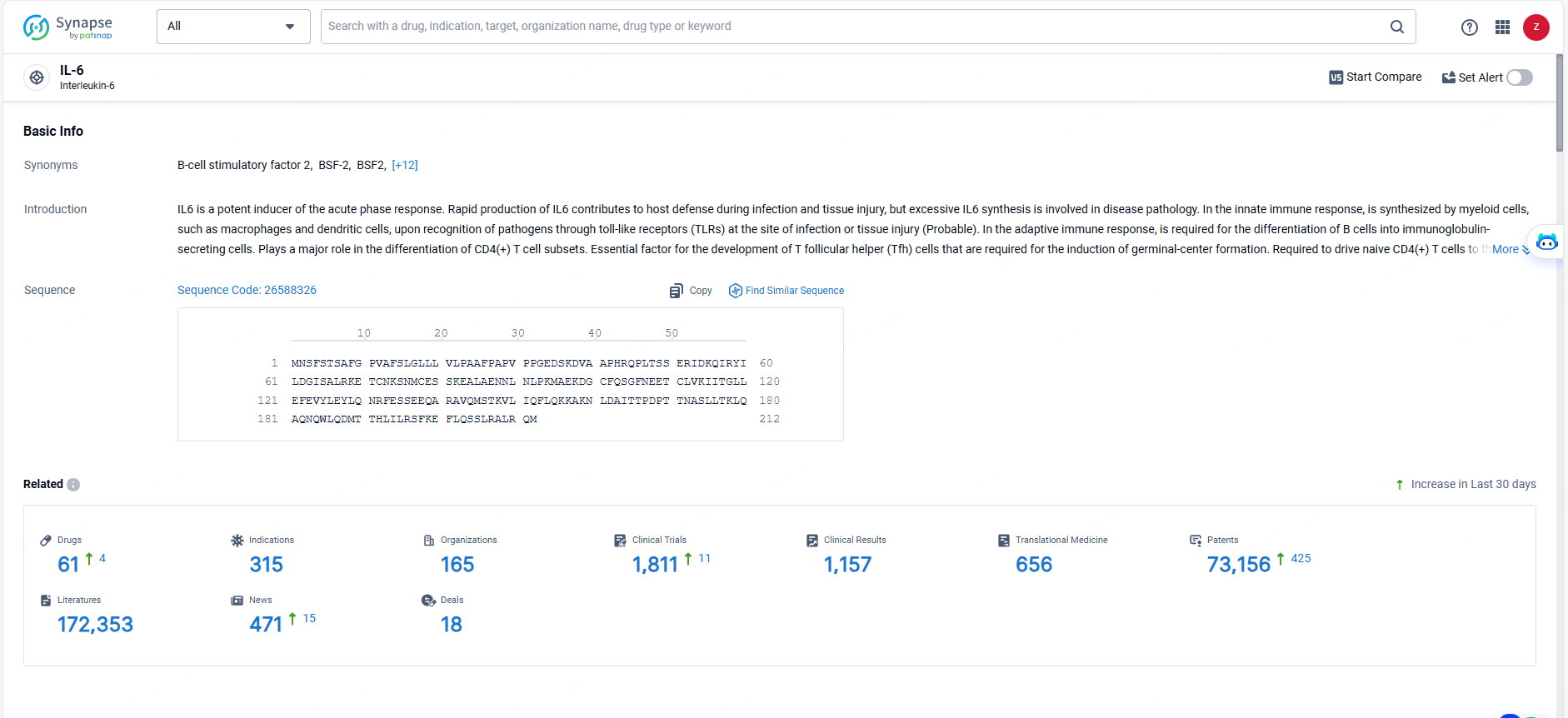
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 61 investigational drugs for the IL-6 targets, including 315 indications, 165 R&D institutions involved, with related clinical trials reaching 1811, and as many as 73156 patents.
TOUR006 is a long-acting, fully human, anti-IL-6 monoclonal antibody with best-in-class potential and differentiated properties, including a naturally long half-life, low immunogenicity, and high binding affinity to IL-6. TOUR006 has been previously studied in 448 participants, including patients with autoimmune disorders, across six completed clinical trials. Tourmaline is developing TOUR006 in thyroid eye disease and atherosclerotic cardiovascular disease as its first two indications, with additional diseases under consideration.