Travere Therapeutics starts key Phase 3 trial of Pegtibatinase for Classical Homocystinuria (HCU) management
Travere Therapeutics, Inc. has declared that it has initiated the recruitment process for its worldwide, pivotal Phase 3 clinical trial named the HARMONY Study. This significant trial is designed to assess the efficacy of pegtibatinase, an innovative enzyme replacement therapy currently under investigation, specifically for the management of classical homocystinuria.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Classical Homocystinuria (HCU) is an infrequent inherited metabolic condition that arises from an insufficiency of the cystathionine beta synthase enzyme. The objective of the investigation is to assess the security and effectiveness of the therapeutic agent pegtibatinase in diminishing the plasma total homocysteine levels, which is a critical aim in the management of classical HCU. This will be measured against a placebo in subjects who are concurrently receiving the standard medical treatment.
"The onset of classical HCU typically occurs in early childhood and can lead to severe health complications due to an excess of the amino acid homocysteine. Possible severe outcomes include a persistent danger of fatal thrombotic incidents, such as myocardial infarctions and cerebrovascular accidents, in addition to structural distortions of the skeleton, developmental hindrances in cognition, and intellectual handicaps," explained Dr. William Rote, the lead for Research and Development at Travere Therapeutics.
Dr. Rote further remarked, "The commencement of the HARMONY Study represents a crucial development in our quest to address this dire clinical need. We are eagerly progressing towards introducing pegtibatinase as a pioneering therapeutic alteration for those affected by classical HCU."
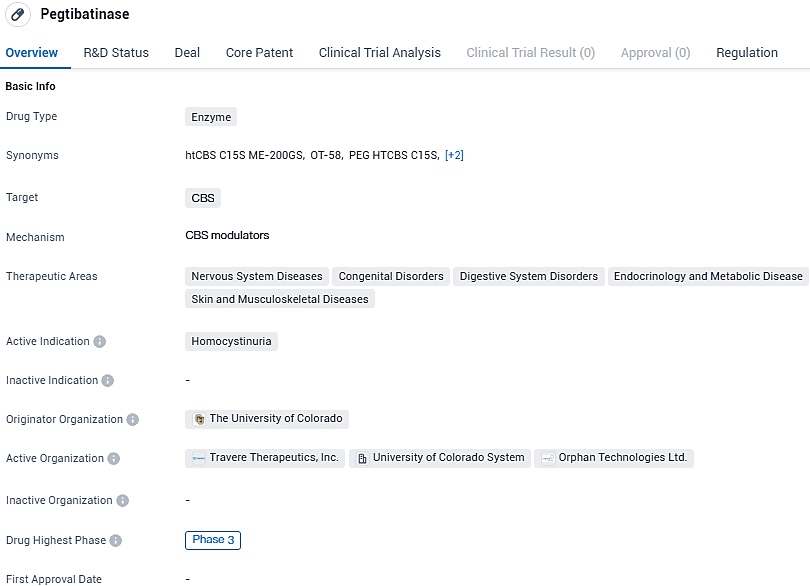
The promising agent pegtibatinase has acquired several endorsements from the U.S. Food and Drug Administration, such as Breakthrough Therapy, Rare Pediatric Disease, and Fast Track designations. It has also received the Orphan Drug status both in the United States and Europe.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
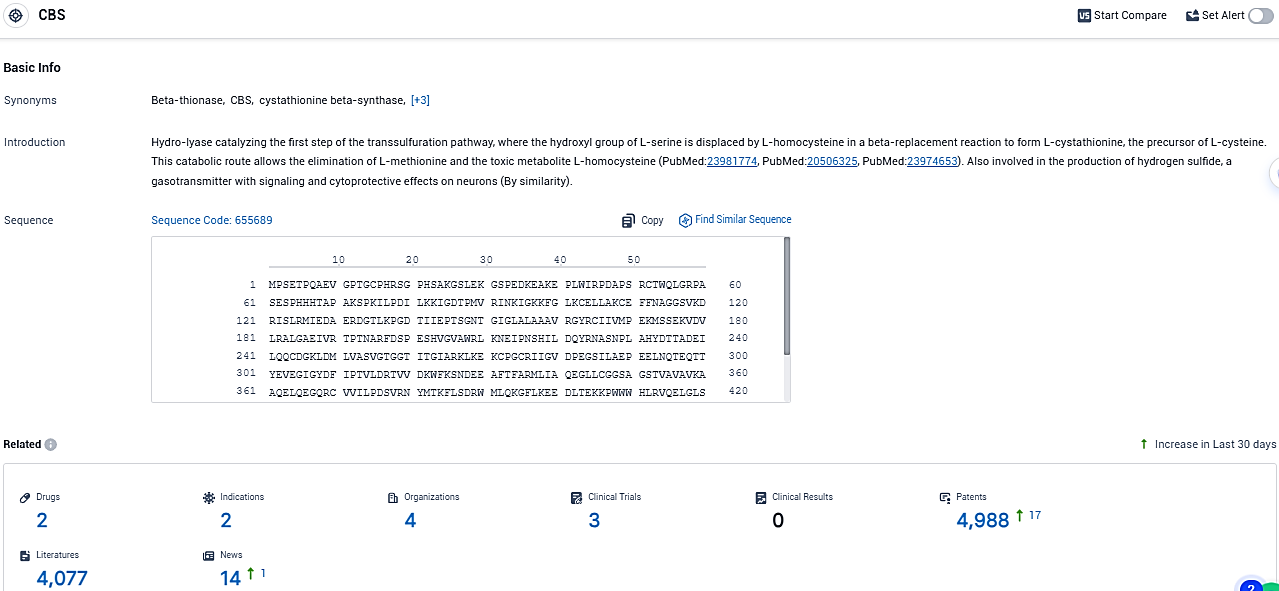
According to the data provided by the Synapse Database, As of December 23, 2023, there are 2 investigational drugs for the CBS target, including 2 indications, 4 R&D institutions involved, with related clinical trials reaching 3, and as many as 4988 patents.
Pegtibatinase is an enzyme-based drug being developed to target CBS and treat homocystinuria. It is currently in Phase 3 of clinical development and has received regulatory designations such as Rare Pediatric Disease, Fast Track, Orphan Drug, and Breakthrough Therapy. These designations highlight the potential of Pegtibatinase to address unmet medical needs and provide improved treatment options for patients with homocys.